Citogenómica en cáncer
Cytogenomics in cancer
Cómo citar
Descargar cita

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-SinDerivadas 4.0.
Mostrar biografía de los autores
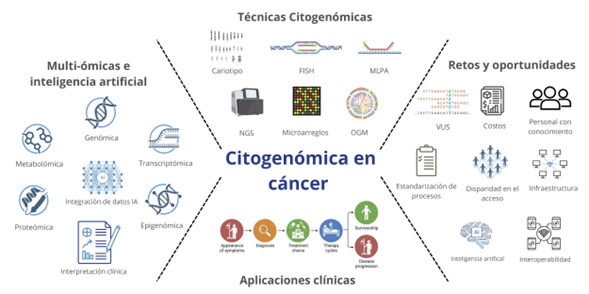
Introducción: la citogenómica combina herramientas citogenéticas clásicas con tecnologías genómicas avanzadas para estudiar alteraciones estructurales del genoma, lo que ha revolucionado el diagnóstico y el tratamiento del cáncer en el contexto de la medicina de precisión.
Métodos: se realizó una revisión narrativa basada en 57 fuentes científicas publicadas entre 2010 y 2025, seleccionadas mediante búsqueda en PubMed, Scopus, Web of Science y Google Scholar. Se incluyeron artículos originales, revisiones, guías clínicas y documentos técnicos. Se utilizaron palabras clave como citogenómica, cáncer, NGS, OGM, medicina de precisión, multiómicas e inteligencia artificial.
Resultados: las técnicas citogenómicas como cariotipo, FISH, MLPA, microarreglos, NGS y mapeo óptico genómico, permiten detectar alteraciones genómicas con valor diagnóstico, pronóstico y terapéutico. La integración con inteligencia artificial mejora la eficiencia y precisión del análisis. Asimismo, los enfoques multiómicos permiten caracterizar mejor los perfiles tumorales y descubrir nuevas dianas terapéuticas. Guías clínicas internacionales y proyectos colaborativos han reforzado su implementación.
Conclusiones: la citogenómica, combinada con la inteligencia artificial y el análisis multiómico, está redefiniendo el abordaje clínico del cáncer. Aunque persisten desafíos técnicos y éticos, su adopción en medicina personalizada continúa en expansión.
Visitas del artículo 0 | Visitas PDF 0
Descargas
- Ribeiro IP, Melo JB, Carreira IM. Cytogenetics and Cytogenomics Evaluation in Cancer. Int J Mol Sci [Internet]. 2019;20(19):4711. Disponible en: https://doi.org/10.3390/ijms20194711
- Chebly A. Cancer cytogenetics in the era of artificial intelligence: shaping the future of chromosome analysis. Future Oncol [Internet]. 2024;20(31):2303-5. Disponible en: https://doi.org/10.1080/14796694.2024.2385296
- Liehr T, editor. Cytogenomics. London San Diego, Calif: Academic Press; [Internet]. 2021. Disponible en: https://doi.org/10.1016/C2020-0-00086-1
- Nowell PC. The minute chromosome (Phl) in chronic granulocytic leukemia. Blut [Internet]. 1962;8(2):65-6. Disponible en: https://doi.org/10.1007/BF01630378
- Bernheim A. Cytogenomics of cancers: From chromosome to sequence. Mol Oncol [Internet]. 2010;4(4):309-22. Disponible en: https://doi.org/10.1016/j.molonc.2010.06.003
- Rowley JD. The critical role of chromosome translocations in human leukemias. Annu Rev Genet [Internet]. 1998;32(1):495-519. Disponible en: https://doi.org/10.1146/annurev.genet.32.1.495
- McGowan-Jordan J, Hastings RJ, Moore S, editores. ISCN 2020: An International System for Human Cytogenomic Nomenclature (2020) [Internet]. Karger; 2020. Disponible en: https://doi.org/10.1159/isbn.978-3-318-06867-2
- Liehr T. From Human Cytogenetics to Human Chromosomics. Int J Mol Sci [Internet]. 2019;20(4):826. Disponible en: https://doi.org/10.3390/ijms20040826
- Casper RM, Leonard K, Mpho M, Bono N, et al. Recent Molecular Techniques in Cytogenetics. Genetics. IntechOpen. [Internet]. 2025. Disponible en: http://dx.doi.org/10.5772/intechopen.1005877
- Durmaz AA, Karaca E, Demkow U, Toruner G, Schoumans J, Cogulu O. Evolution of Genetic Techniques: Past, Present, and Beyond. BioMed Res Int [Internet]. 2015;2015:461524. Disponible en: https://doi.org/10.1155/2015/461524
- Alhussain AH, Alquwayi WA, Alkuwaiti YAA, Almehainy AM, Alkhathami AA. Applications of Cytogenetics and Cytogenomics Evaluation techniques in cancer diagnosis: A review. Int J Health Sci [Internet]. 2019;3(S1):336-51. Disponible en: https://doi.org/10.53730/ijhs.v3ns1.15214
- Balciuniene J, Ning Y, Lazarus HM, et al. Cancer cytogenetics in a genomics world: Wedding the old with the new. Blood Rev [Internet]. 2024;66:101209. Disponible en: https://doi.org/10.1016/j.blre.2024.101209
- Nath J, Johnson KL. A Review of Fluorescence in Situ Hybridization (FISH): Current Status and Future Prospects. Biotech Histochem [Internet]. 2000;75(2):54-78. Disponible en: https://doi.org/10.3109/10520290009064150
- Marina AM. Fluorescence in situ hybridization (FISH). En: Arsham MS, Barch MJ, Lawce HJ, editores. The AGT Cytogenetics Laboratory Manual [Internet]. 4.a ed. Wiley; 2017. p. 717-831. Disponible en: https://doi.org/10.1002/9781119061199.ch16
- Ha J, Cho H, Lee TG, et al. Cytogenetic testing by fluorescence in situ hybridization is improved by plasma cell sorting in multiple myeloma. Sci Rep [Internet]. 2022;12(1):8287. Disponible en: https://doi.org/10.1038/s41598-022-11676-w
- Davé BJ, Sanger WG. Genomic microarray technologies for the cytogenetics laboratory. En: The AGT Cytogenetics Laboratory Manual [Internet]. Wiley; 2017. p. 903-36. Disponible en: https://doi.org/10.1002/9781119061199.ch18
- Heller MJ. DNA Microarray Technology: Devices, Systems, and Applications. Annu Rev Biomed Eng [Internet]. 2002;4:129-53. Disponible en: https://doi.org/10.1146/annurev.bioeng.4.020702.153438
- Bumgarner R. Overview of DNA Microarrays: Types, Applications, and Their Future. Curr Protoc Mol Biol [Internet]. 2013;101(1):22.1.1-22.1.11. Disponible en: https://doi.org/10.1002/0471142727.mb2201s101
- Wang Y, Liehr T. The Need for a Concert of Cytogenomic Methods in Chromosomic Research and Diagnostics. Genes [Internet]. 2025;16(5):533. Disponible en: https://doi.org/10.3390/genes16050533
- Rack KA, van den Berg E, Haferlach C, et al. European recommendations and quality assurance for cytogenomic analysis of haematological neoplasms. Leukemia [Internet]. 2019;33:1851-67. Disponible en: https://doi.org/10.1038/s41375-019-0378-z
- Hu T, Chitnis N, Monos D, Dinh A. Next-generation sequencing technologies: An overview. Hum Immunol [Internet]. 2021;82(11):801-11. Disponible en: https://doi.org/10.1016/j.humimm.2021.02.012
- Cheng C, Fei Z, Xiao P. Methods to improve the accuracy of next-generation sequencing. Front Bioeng Biotechnol [Internet]. 2023;11:982111. Disponible en: https://doi.org/10.3389/fbioe.2023.982111
- Mosele MF, Westphalen CB, Stenzinger A, et al. Recommendations for the use of next-generation sequencing (NGS) for patients with advanced cancer in 2024: a report from the ESMO Precision Medicine Working Group. Ann Oncol [Internet]. 2024;35(7):588-606. Disponible en: https://doi.org/10.1016/j.annonc.2024.04.005
- Satam H, Joshi K, Mangrolia U, et al. Next-Generation Sequencing Technology: Current Trends and Advancements. Biology [Internet]. 2023;12(7):997. Disponible en: https://doi.org/10.3390/biology12070997
- Yohe S, Thyagarajan B. Review of Clinical Next-Generation Sequencing. Arch Pathol Lab Med [Internet]. 2017;141(11):1544-57. Disponible en: https://doi.org/10.5858/arpa.2016-0501-RA
- Slatko BE, Gardner AF, Ausubel FM. Overview of Next-Generation Sequencing Technologies. Curr Protoc Mol Biol [Internet]. 2018;122(1):e59. Disponible en: https://doi.org/10.1002/cpmb.59
- Dey SK. Technological Advances in Cancer Cytogenetics. Genetics. IntechOpen [Internet]. 2024. Disponible en: http://dx.doi.org/10.5772/intechopen.1008240
- Gerding WM, Tembrink M, Nilius-Eliliwi V, et al. Optical genome mapping reveals additional prognostic information compared to conventional cytogenetics in AML/MDS patients. Int J Cancer [Internet]. 2022;150(12):1998-2011. Disponible en: https://doi.org/10.1002/ijc.33942
- Barseghyan H, Eisenreich D, Lindt E, et al. Optical Genome Mapping as a Potential Routine Clinical Diagnostic Method. Genes [Internet]. 2024;15(3):342. Disponible en: https://doi.org/10.3390/genes15030342
- Schrauwen I, Rajendran Y, Acharya A, et al. Optical genome mapping unveils hidden structural variants in neurodevelopmental disorders. Sci Rep [Internet]. 2024;14(1):11239. Disponible en: https://doi.org/10.1038/s41598-024-62009-y
- Sahajpal NS, Mondal AK, Fee T, et al. Clinical Validation and Diagnostic Utility of Optical Genome Mapping in Prenatal Diagnostic Testing. J Mol Diagn [Internet]. 2023;25(4):234-46. Disponible en: https://doi.org/10.1016/j.jmoldx.2023.01.006
- Zou YS, Klausner M, Ghabrial J, et al. A comprehensive approach to evaluate genetic abnormalities in multiple myeloma using optical genome mapping. Blood Cancer J [Internet]. 2024;14(1):78. Disponible en: https://doi.org/10.1038/s41408-024-01059-x
- Kanagal-Shamanna R, Puiggros A, Granada I, et al. Integration of Optical Genome Mapping in the Cytogenomic and Molecular Work-Up of Hematological Malignancies: Expert Recommendations From the International Consortium for Optical Genome Mapping. Am J Hematol [Internet]. 2025;100(6):1029-48. Disponible en: https://doi.org/10.1002/ajh.27688
- Ballesta-Alcaraz L, Bernal M, Vilchez JR, et al. Application of Optical Genome Mapping for the Diagnosis and Risk Stratification of Myeloid and Lymphoid Malignancies. Int J Mol Sci [Internet]. 2025;26(12):5763. Disponible en: https://doi.org/10.3390/ijms26125763
- Levy B, Baughn LB, Akkari Y, et al. Optical genome mapping in acute myeloid leukemia: a multicenter evaluation. Blood Adv [Internet]. 2023;7(7):1297-307. Disponible en: https://doi.org/10.1182/bloodadvances.2022007583
- Shim Y, Koo YK, Shin S, et al. Comparison of Optical Genome Mapping With Conventional Diagnostic Methods for Structural Variant Detection in Hematologic Malignancies. Ann Lab Med [Internet]. 2024;44(4):324-34. Disponible en: https://doi.org/10.3343/alm.2023.0339
- Tsai MJM, Kao HJ, Chen HH, et al. Optical genome mapping with whole genome sequencing identifies complex chromosomal structural variations in acute leukemia. Front Genet [Internet]. 2025;16:1496847. Disponible en: https://doi.org/10.3389/fgene.2025.1496847
- Lestringant V, Guermouche-Flament H, Jimenez-Pocquet M, et al. Cytogenetics in the management of hematological malignancies: An overview of alternative technologies for cytogenetic characterization. Curr Res Transl Med [Internet]. 2024;72(3):103440. Disponible en: https://doi.org/10.1016/j.retram.2024.103440
- Toruner GA, Hu S, Loghavi S, et al. Clinical Utility of Optical Genome Mapping as an Additional Tool in a Standard Cytogenetic Workup in Hematological Malignancies. Cancers [Internet]. 2025;17(9):1436. Disponible en: https://doi.org/10.3390/cancers17091436
- Singh G, Kamalja A, Patil R, et al. A comprehensive assessment of artificial intelligence applications for cancer diagnosis. Artif Intell Rev [Internet]. 2024;57(7):179. Disponible en: https://doi.org/10.1007/s10462-024-10783-6
- Maqsood K, Hagras H, Zabet NR. An overview of artificial intelligence in the field of genomics. Discov Artif Intell [Internet]. 2024;4(1):9. Disponible en: https://doi.org/10.1007/s44163-024-00103-w
- Jeziorski K, Olszewski R. Artificial Intelligence in Oncology. Appl Sci [Internet]. 2025;15(1):269. Disponible en: https://doi.org/10.3390/app15010269
- Walter W, Haferlach C, Nadarajah N, et al. How artificial intelligence might disrupt diagnostics in hematology in the near future. Oncogene [Internet]. 2021;40(25):4271-80. Disponible en: https://doi.org/10.1038/s41388-021-01861-y
- Duong D, Solomon BD. Artificial intelligence in clinical genetics. Eur J Hum Genet [Internet]. 2025;33(3):281-8. Disponible en: https://doi.org/10.1038/s41431-024-01782-w
- Zhou Y, Xu L, Zhang L, Shi D, Wu C, Wei R, et al. Enhancing chromosomal analysis efficiency through deep learning-based artificial intelligence graphic analysis. Discov Appl Sci [Internet]. 2024;6:299. Disponible en: https://doi.org/10.1007/s42452-024-05980-5
- Rosenblum LS, Holmes J, Taghiyev AF. The Emergence of Artificial Intelligence-Guided Karyotyping: A Review and Reflection. Genes [Internet]. 2025;16(6):685. Disponible en: https://doi.org/10.3390/genes16060685
- Felici A, Peduzzi G, Pellungrini R, Campa D. Artificial intelligence to predict cancer risk, are we there yet? A comprehensive review across cancer types. Eur J Cancer [Internet]. 2025;228:115716. Disponible en: https://doi.org/10.1016/j.ejca.2025.115716
- Macheka S, Ng PY, Ginsburg O, et al. Prospective evaluation of artificial intelligence (AI) applications for use in cancer pathways following diagnosis: a systematic review. BMJ Oncol [Internet]. 2024;3(1):e000255. Disponible en: https://doi.org/10.1136/bmjonc-2023-000255
- Shirazi AZ, Tofighi M, Gharavi A, Gomez GA. The Application of Artificial Intelligence to Cancer Research: A Comprehensive Guide. Cancer Control [Internet]. 2024;31:15330338241250324. Disponible en: https://doi.org/10.1177/15330338241250324
- Mani S, Lalani SR, Pammi M. Genomics and multiomics in the age of precision medicine. Pediatr Res [Internet]. 2025;97:1399-1410. Disponible en: https://doi.org/10.1038/s41390-025-04021-0
- Zack M, Stupichev DN, Moore AJ, et al. AI and Multi-Omics in Pharmacogenomics: A New Era of Precision Medicine. Mayo Clin Proc Digit Health [Internet]. 2025;3(3):100246. Disponible en: https://doi.org/10.1016/j.mcpdig.2025.100246
- Shao Y, Lv X, Ying S, Guo Q. Artificial Intelligence-Driven Precision Medicine: Multi-Omics and Spatial Multi-Omics Approaches in Diffuse Large B-Cell Lymphoma (DLBCL). Front Biosci-Landmark [Internet]. 2024;29(12):404. Disponible en: https://doi.org/10.31083/j.fbl2912404
- Kioroglou D, Gil-Redondo R, Embade N, et al. Multi-omic integration sets the path for early prevention strategies on healthy individuals. Npj Genomic Med [Internet]. 2025;10:35. Disponible en: https://doi.org/10.1038/s41525-025-00491-7
- Raufaste-Cazavieille V, Santiago R, Droit A. Multi-omics analysis: Paving the path toward achieving precision medicine in cancer treatment and immuno-oncology. Front Mol Biosci [Internet]. 2022;9:962743. Disponible en: https://doi.org/10.3389/fmolb.2022.962743
- Ikwelle TA, Ihim AC, Ozuruoke DFN, et al. Multi-Omics Integration in Personalized Medicine: Advancing Laboratory Diagnostics and Precision Therapeutics in the Era of Individualized Healthcare. J Drug Deliv Ther [Internet]. 2025;15(5):132-42. Disponible en: https://doi.org/10.22270/jddt.v15i5.7121
- Molla G, Bitew M. Revolutionizing Personalized Medicine: Synergy with Multi-Omics Data generation, Main Hurdles, and Future Perspectives. Biomedicines [Internet]. 2024;12(12):2750. Disponible en: https://doi.org/10.3390/biomedicines12122750
- Mohr AE, Ortega-Santos CP, Whisner CM, et al. Navigating Challenges and Opportunities in Multi-Omics Integration for Personalized Healthcare. Biomedicines [Internet]. 2024;12(7):1496. Disponible en: https://doi.org/10.3390/biomedicines12071496
- Shimony S, Stahl M, Stone RM. Acute Myeloid Leukemia: 2025 Update on Diagnosis, Risk-Stratification, and Management. Am J Hematol [Internet]. 2025;100(5):860-91. Disponible en: https://doi.org/10.1002/ajh.27625

















