Cytogenomics in cancer
Citogenómica en cáncer
How to Cite
Download Citation

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Show authors biography
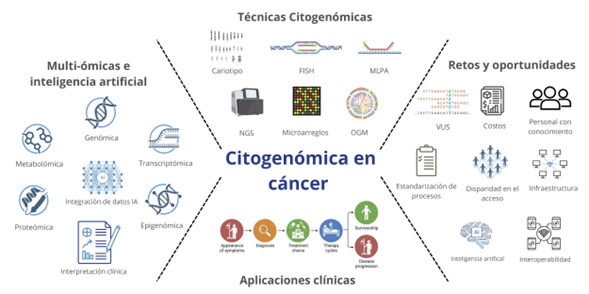
Introduction: cytogenomics integrates classical cytogenetics with advanced genomic tools to identify structural genome alterations, reshaping cancer diagnosis and treatment within precision medicine.
Methods: a narrative review was conducted using 57 sources from 2010 to 2025, retrieved from PubMed, Scopus, Web of Science, and Google Scholar. Included materials were original articles, reviews, clinical guidelines, and technical documents. Keywords included cytogenomics, cancer, NGS, OGM, precision medicine, multi-omics, and artificial intelligence.
Results: techniques such as karyotyping, FISH, MLPA, microarrays, NGS, and optical genome mapping enable high-resolution detection of clinically relevant genomic alterations. Integration with artificial intelligence improves analytic accuracy. Multiomic strategies enhance tumor profiling and discovery of novel therapeutic targets. Clinical guidelines and international initiatives support clinical adoption.
Conclusions: cytogenomics, strengthened by AI and multi-omics, is transforming the clinical management of cancer. Despite technological and ethical challenges, its role in personalized oncology continues to expand.
Article visits 0 | PDF visits 0
Downloads
- Ribeiro IP, Melo JB, Carreira IM. Cytogenetics and Cytogenomics Evaluation in Cancer. Int J Mol Sci [Internet]. 2019;20(19):4711. Disponible en: https://doi.org/10.3390/ijms20194711
- Chebly A. Cancer cytogenetics in the era of artificial intelligence: shaping the future of chromosome analysis. Future Oncol [Internet]. 2024;20(31):2303-5. Disponible en: https://doi.org/10.1080/14796694.2024.2385296
- Liehr T, editor. Cytogenomics. London San Diego, Calif: Academic Press; [Internet]. 2021. Disponible en: https://doi.org/10.1016/C2020-0-00086-1
- Nowell PC. The minute chromosome (Phl) in chronic granulocytic leukemia. Blut [Internet]. 1962;8(2):65-6. Disponible en: https://doi.org/10.1007/BF01630378
- Bernheim A. Cytogenomics of cancers: From chromosome to sequence. Mol Oncol [Internet]. 2010;4(4):309-22. Disponible en: https://doi.org/10.1016/j.molonc.2010.06.003
- Rowley JD. The critical role of chromosome translocations in human leukemias. Annu Rev Genet [Internet]. 1998;32(1):495-519. Disponible en: https://doi.org/10.1146/annurev.genet.32.1.495
- McGowan-Jordan J, Hastings RJ, Moore S, editores. ISCN 2020: An International System for Human Cytogenomic Nomenclature (2020) [Internet]. Karger; 2020. Disponible en: https://doi.org/10.1159/isbn.978-3-318-06867-2
- Liehr T. From Human Cytogenetics to Human Chromosomics. Int J Mol Sci [Internet]. 2019;20(4):826. Disponible en: https://doi.org/10.3390/ijms20040826
- Casper RM, Leonard K, Mpho M, Bono N, et al. Recent Molecular Techniques in Cytogenetics. Genetics. IntechOpen. [Internet]. 2025. Disponible en: http://dx.doi.org/10.5772/intechopen.1005877
- Durmaz AA, Karaca E, Demkow U, Toruner G, Schoumans J, Cogulu O. Evolution of Genetic Techniques: Past, Present, and Beyond. BioMed Res Int [Internet]. 2015;2015:461524. Disponible en: https://doi.org/10.1155/2015/461524
- Alhussain AH, Alquwayi WA, Alkuwaiti YAA, Almehainy AM, Alkhathami AA. Applications of Cytogenetics and Cytogenomics Evaluation techniques in cancer diagnosis: A review. Int J Health Sci [Internet]. 2019;3(S1):336-51. Disponible en: https://doi.org/10.53730/ijhs.v3ns1.15214
- Balciuniene J, Ning Y, Lazarus HM, et al. Cancer cytogenetics in a genomics world: Wedding the old with the new. Blood Rev [Internet]. 2024;66:101209. Disponible en: https://doi.org/10.1016/j.blre.2024.101209
- Nath J, Johnson KL. A Review of Fluorescence in Situ Hybridization (FISH): Current Status and Future Prospects. Biotech Histochem [Internet]. 2000;75(2):54-78. Disponible en: https://doi.org/10.3109/10520290009064150
- Marina AM. Fluorescence in situ hybridization (FISH). En: Arsham MS, Barch MJ, Lawce HJ, editores. The AGT Cytogenetics Laboratory Manual [Internet]. 4.a ed. Wiley; 2017. p. 717-831. Disponible en: https://doi.org/10.1002/9781119061199.ch16
- Ha J, Cho H, Lee TG, et al. Cytogenetic testing by fluorescence in situ hybridization is improved by plasma cell sorting in multiple myeloma. Sci Rep [Internet]. 2022;12(1):8287. Disponible en: https://doi.org/10.1038/s41598-022-11676-w
- Davé BJ, Sanger WG. Genomic microarray technologies for the cytogenetics laboratory. En: The AGT Cytogenetics Laboratory Manual [Internet]. Wiley; 2017. p. 903-36. Disponible en: https://doi.org/10.1002/9781119061199.ch18
- Heller MJ. DNA Microarray Technology: Devices, Systems, and Applications. Annu Rev Biomed Eng [Internet]. 2002;4:129-53. Disponible en: https://doi.org/10.1146/annurev.bioeng.4.020702.153438
- Bumgarner R. Overview of DNA Microarrays: Types, Applications, and Their Future. Curr Protoc Mol Biol [Internet]. 2013;101(1):22.1.1-22.1.11. Disponible en: https://doi.org/10.1002/0471142727.mb2201s101
- Wang Y, Liehr T. The Need for a Concert of Cytogenomic Methods in Chromosomic Research and Diagnostics. Genes [Internet]. 2025;16(5):533. Disponible en: https://doi.org/10.3390/genes16050533
- Rack KA, van den Berg E, Haferlach C, et al. European recommendations and quality assurance for cytogenomic analysis of haematological neoplasms. Leukemia [Internet]. 2019;33:1851-67. Disponible en: https://doi.org/10.1038/s41375-019-0378-z
- Hu T, Chitnis N, Monos D, Dinh A. Next-generation sequencing technologies: An overview. Hum Immunol [Internet]. 2021;82(11):801-11. Disponible en: https://doi.org/10.1016/j.humimm.2021.02.012
- Cheng C, Fei Z, Xiao P. Methods to improve the accuracy of next-generation sequencing. Front Bioeng Biotechnol [Internet]. 2023;11:982111. Disponible en: https://doi.org/10.3389/fbioe.2023.982111
- Mosele MF, Westphalen CB, Stenzinger A, et al. Recommendations for the use of next-generation sequencing (NGS) for patients with advanced cancer in 2024: a report from the ESMO Precision Medicine Working Group. Ann Oncol [Internet]. 2024;35(7):588-606. Disponible en: https://doi.org/10.1016/j.annonc.2024.04.005
- Satam H, Joshi K, Mangrolia U, et al. Next-Generation Sequencing Technology: Current Trends and Advancements. Biology [Internet]. 2023;12(7):997. Disponible en: https://doi.org/10.3390/biology12070997
- Yohe S, Thyagarajan B. Review of Clinical Next-Generation Sequencing. Arch Pathol Lab Med [Internet]. 2017;141(11):1544-57. Disponible en: https://doi.org/10.5858/arpa.2016-0501-RA
- Slatko BE, Gardner AF, Ausubel FM. Overview of Next-Generation Sequencing Technologies. Curr Protoc Mol Biol [Internet]. 2018;122(1):e59. Disponible en: https://doi.org/10.1002/cpmb.59
- Dey SK. Technological Advances in Cancer Cytogenetics. Genetics. IntechOpen [Internet]. 2024. Disponible en: http://dx.doi.org/10.5772/intechopen.1008240
- Gerding WM, Tembrink M, Nilius-Eliliwi V, et al. Optical genome mapping reveals additional prognostic information compared to conventional cytogenetics in AML/MDS patients. Int J Cancer [Internet]. 2022;150(12):1998-2011. Disponible en: https://doi.org/10.1002/ijc.33942
- Barseghyan H, Eisenreich D, Lindt E, et al. Optical Genome Mapping as a Potential Routine Clinical Diagnostic Method. Genes [Internet]. 2024;15(3):342. Disponible en: https://doi.org/10.3390/genes15030342
- Schrauwen I, Rajendran Y, Acharya A, et al. Optical genome mapping unveils hidden structural variants in neurodevelopmental disorders. Sci Rep [Internet]. 2024;14(1):11239. Disponible en: https://doi.org/10.1038/s41598-024-62009-y
- Sahajpal NS, Mondal AK, Fee T, et al. Clinical Validation and Diagnostic Utility of Optical Genome Mapping in Prenatal Diagnostic Testing. J Mol Diagn [Internet]. 2023;25(4):234-46. Disponible en: https://doi.org/10.1016/j.jmoldx.2023.01.006
- Zou YS, Klausner M, Ghabrial J, et al. A comprehensive approach to evaluate genetic abnormalities in multiple myeloma using optical genome mapping. Blood Cancer J [Internet]. 2024;14(1):78. Disponible en: https://doi.org/10.1038/s41408-024-01059-x
- Kanagal-Shamanna R, Puiggros A, Granada I, et al. Integration of Optical Genome Mapping in the Cytogenomic and Molecular Work-Up of Hematological Malignancies: Expert Recommendations From the International Consortium for Optical Genome Mapping. Am J Hematol [Internet]. 2025;100(6):1029-48. Disponible en: https://doi.org/10.1002/ajh.27688
- Ballesta-Alcaraz L, Bernal M, Vilchez JR, et al. Application of Optical Genome Mapping for the Diagnosis and Risk Stratification of Myeloid and Lymphoid Malignancies. Int J Mol Sci [Internet]. 2025;26(12):5763. Disponible en: https://doi.org/10.3390/ijms26125763
- Levy B, Baughn LB, Akkari Y, et al. Optical genome mapping in acute myeloid leukemia: a multicenter evaluation. Blood Adv [Internet]. 2023;7(7):1297-307. Disponible en: https://doi.org/10.1182/bloodadvances.2022007583
- Shim Y, Koo YK, Shin S, et al. Comparison of Optical Genome Mapping With Conventional Diagnostic Methods for Structural Variant Detection in Hematologic Malignancies. Ann Lab Med [Internet]. 2024;44(4):324-34. Disponible en: https://doi.org/10.3343/alm.2023.0339
- Tsai MJM, Kao HJ, Chen HH, et al. Optical genome mapping with whole genome sequencing identifies complex chromosomal structural variations in acute leukemia. Front Genet [Internet]. 2025;16:1496847. Disponible en: https://doi.org/10.3389/fgene.2025.1496847
- Lestringant V, Guermouche-Flament H, Jimenez-Pocquet M, et al. Cytogenetics in the management of hematological malignancies: An overview of alternative technologies for cytogenetic characterization. Curr Res Transl Med [Internet]. 2024;72(3):103440. Disponible en: https://doi.org/10.1016/j.retram.2024.103440
- Toruner GA, Hu S, Loghavi S, et al. Clinical Utility of Optical Genome Mapping as an Additional Tool in a Standard Cytogenetic Workup in Hematological Malignancies. Cancers [Internet]. 2025;17(9):1436. Disponible en: https://doi.org/10.3390/cancers17091436
- Singh G, Kamalja A, Patil R, et al. A comprehensive assessment of artificial intelligence applications for cancer diagnosis. Artif Intell Rev [Internet]. 2024;57(7):179. Disponible en: https://doi.org/10.1007/s10462-024-10783-6
- Maqsood K, Hagras H, Zabet NR. An overview of artificial intelligence in the field of genomics. Discov Artif Intell [Internet]. 2024;4(1):9. Disponible en: https://doi.org/10.1007/s44163-024-00103-w
- Jeziorski K, Olszewski R. Artificial Intelligence in Oncology. Appl Sci [Internet]. 2025;15(1):269. Disponible en: https://doi.org/10.3390/app15010269
- Walter W, Haferlach C, Nadarajah N, et al. How artificial intelligence might disrupt diagnostics in hematology in the near future. Oncogene [Internet]. 2021;40(25):4271-80. Disponible en: https://doi.org/10.1038/s41388-021-01861-y
- Duong D, Solomon BD. Artificial intelligence in clinical genetics. Eur J Hum Genet [Internet]. 2025;33(3):281-8. Disponible en: https://doi.org/10.1038/s41431-024-01782-w
- Zhou Y, Xu L, Zhang L, Shi D, Wu C, Wei R, et al. Enhancing chromosomal analysis efficiency through deep learning-based artificial intelligence graphic analysis. Discov Appl Sci [Internet]. 2024;6:299. Disponible en: https://doi.org/10.1007/s42452-024-05980-5
- Rosenblum LS, Holmes J, Taghiyev AF. The Emergence of Artificial Intelligence-Guided Karyotyping: A Review and Reflection. Genes [Internet]. 2025;16(6):685. Disponible en: https://doi.org/10.3390/genes16060685
- Felici A, Peduzzi G, Pellungrini R, Campa D. Artificial intelligence to predict cancer risk, are we there yet? A comprehensive review across cancer types. Eur J Cancer [Internet]. 2025;228:115716. Disponible en: https://doi.org/10.1016/j.ejca.2025.115716
- Macheka S, Ng PY, Ginsburg O, et al. Prospective evaluation of artificial intelligence (AI) applications for use in cancer pathways following diagnosis: a systematic review. BMJ Oncol [Internet]. 2024;3(1):e000255. Disponible en: https://doi.org/10.1136/bmjonc-2023-000255
- Shirazi AZ, Tofighi M, Gharavi A, Gomez GA. The Application of Artificial Intelligence to Cancer Research: A Comprehensive Guide. Cancer Control [Internet]. 2024;31:15330338241250324. Disponible en: https://doi.org/10.1177/15330338241250324
- Mani S, Lalani SR, Pammi M. Genomics and multiomics in the age of precision medicine. Pediatr Res [Internet]. 2025;97:1399-1410. Disponible en: https://doi.org/10.1038/s41390-025-04021-0
- Zack M, Stupichev DN, Moore AJ, et al. AI and Multi-Omics in Pharmacogenomics: A New Era of Precision Medicine. Mayo Clin Proc Digit Health [Internet]. 2025;3(3):100246. Disponible en: https://doi.org/10.1016/j.mcpdig.2025.100246
- Shao Y, Lv X, Ying S, Guo Q. Artificial Intelligence-Driven Precision Medicine: Multi-Omics and Spatial Multi-Omics Approaches in Diffuse Large B-Cell Lymphoma (DLBCL). Front Biosci-Landmark [Internet]. 2024;29(12):404. Disponible en: https://doi.org/10.31083/j.fbl2912404
- Kioroglou D, Gil-Redondo R, Embade N, et al. Multi-omic integration sets the path for early prevention strategies on healthy individuals. Npj Genomic Med [Internet]. 2025;10:35. Disponible en: https://doi.org/10.1038/s41525-025-00491-7
- Raufaste-Cazavieille V, Santiago R, Droit A. Multi-omics analysis: Paving the path toward achieving precision medicine in cancer treatment and immuno-oncology. Front Mol Biosci [Internet]. 2022;9:962743. Disponible en: https://doi.org/10.3389/fmolb.2022.962743
- Ikwelle TA, Ihim AC, Ozuruoke DFN, et al. Multi-Omics Integration in Personalized Medicine: Advancing Laboratory Diagnostics and Precision Therapeutics in the Era of Individualized Healthcare. J Drug Deliv Ther [Internet]. 2025;15(5):132-42. Disponible en: https://doi.org/10.22270/jddt.v15i5.7121
- Molla G, Bitew M. Revolutionizing Personalized Medicine: Synergy with Multi-Omics Data generation, Main Hurdles, and Future Perspectives. Biomedicines [Internet]. 2024;12(12):2750. Disponible en: https://doi.org/10.3390/biomedicines12122750
- Mohr AE, Ortega-Santos CP, Whisner CM, et al. Navigating Challenges and Opportunities in Multi-Omics Integration for Personalized Healthcare. Biomedicines [Internet]. 2024;12(7):1496. Disponible en: https://doi.org/10.3390/biomedicines12071496
- Shimony S, Stahl M, Stone RM. Acute Myeloid Leukemia: 2025 Update on Diagnosis, Risk-Stratification, and Management. Am J Hematol [Internet]. 2025;100(5):860-91. Disponible en: https://doi.org/10.1002/ajh.27625

















