Biología molecular del cáncer epitelial de ovario
Molecular biology of epithelial ovarian cancer
Cómo citar
Descargar cita

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-SinDerivadas 4.0.
Mostrar biografía de los autores
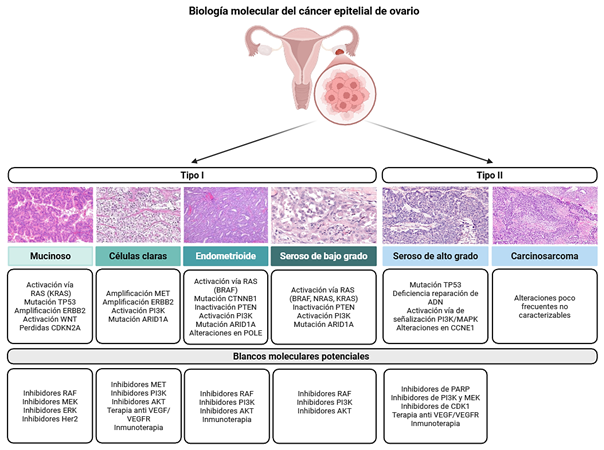
Introducción: el cáncer epitelial de ovario es una neoplasia heterogénea y de alta mortalidad, caracterizada por diversos subtipos histológicos y moleculares que condicionan su comportamiento clínico, pronóstico y respuesta terapéutica. El carcinoma seroso de alto grado es el subtipo más frecuente y se asocia a diagnóstico en estadios avanzados y elevada tasa de recurrencia.
Métodos: se realizó una revisión narrativa de la literatura científica sobre los aspectos histopatológicos, moleculares y terapéuticos del cáncer epitelial de ovario, con énfasis en la caracterización molecular, los avances en terapias dirigidas y el papel emergente de la biopsia líquida.
Resultados: el carcinoma seroso de alto grado presenta una marcada inestabilidad genómica, mutaciones en TP53 y alteraciones en la vía de recombinación homóloga, especialmente mutaciones germinales o somáticas en BRCA1/2, lo que ha permitido la incorporación de inhibidores de PARP como estrategia terapéutica. Otros subtipos menos frecuentes (endometrioide, de células claras, mucinoso y seroso de bajo grado) exhiben perfiles moleculares distintos, con mutaciones recurrentes en ARID1A, PIK3CA, KRAS o CTNNB1, que tienen implicaciones pronósticas y terapéuticas específicas. La clasificación transcriptómica propuesta por The Cancer Genome Atlas (TCGA) identifica subtipos inmunorreactivo, mesenquimal, proliferativo y diferenciado, reflejando la complejidad biológica del tumor. Asimismo, las estrategias de mantenimiento con terapias dirigidas, como bevacizumab y los inhibidores de PARP (olaparib, niraparib y rucaparib), han demostrado mejorar los desenlaces en pacientes seleccionadas. La biopsia líquida emerge como una herramienta prometedora para el monitoreo dinámico de la enfermedad y la detección temprana de recurrencias.
Conclusiones: la integración de la información clínica, molecular y del microambiente tumoral es fundamental para avanzar hacia un manejo verdaderamente personalizado del cáncer epitelial de ovario, optimizando la selección terapéutica y mejorando los resultados clínicos.
Visitas del artículo 0 | Visitas PDF 0
Descargas
- Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. [Internet] 2024;74(3):229-263. Disponible en: https://doi.org/10.3322/caac.21834
- Wang M, Bi Y, Jin Y, Zheng ZJ. Global Incidence of Ovarian Cancer According to Histologic Subtype: A Population-Based Cancer Registry Study. JCO Glob Oncol. [Internet] 2024;10:e2300393. Disponible en: https://doi.org/10.1200/go.23.00393
- Rojas V, Hirshfield KM, Ganesan S, Rodriguez-Rodriguez L. Molecular Characterization of Epithelial Ovarian Cancer: Implications for Diagnosis and Treatment. Int J Mol Sci. [Internet] 2016;17(12). Disponible en: https://doi.org/10.3390/ijms17122113
- Soovares P, Pasanen A, Similä-Maarala J, Bützow R, Lassus H. Clinical factors and biomarker profiles associated with patient outcome in endometrioid ovarian carcinoma - Emphasis on tumor grade. Gynecol Oncol. [Internet] 2022;164(1):187-194. Disponible en: https://doi.org/10.1016/j.ygyno.2021.10.078
- Köbel M, Rahimi K, Rambau PF, et al. An Immunohistochemical Algorithm for Ovarian Carcinoma Typing. Int J Gynecol Pathol. [Internet] 2016;35(5):430-41. Disponible en: https://doi.org/10.1097/pgp.0000000000000274
- Sieh W, Köbel M, Longacre TA, et al. Hormone-receptor expression and ovarian cancer survival: an Ovarian Tumor Tissue Analysis consortium study. Lancet Oncol. [Internet] 2013;14(9):853-62. Disponible en: https://doi.org/10.1016/s1470-2045(13)70253-5
- McCluggage WG. Morphological subtypes of ovarian carcinoma: a review with emphasis on new developments and pathogenesis. Pathology. [Internet] 2011;43(5):420-32. Disponible en: https://doi.org/10.1097/PAT.0b013e328348a6e7
- Hollis RL, Thomson JP, Stanley B, et al. Molecular stratification of endometrioid ovarian carcinoma predicts clinical outcome. Nat Commun. [Internet] 2020;11(1):4995. Disponible en: https://doi.org/10.1038/s41467-020-18819-5
- Parra-Herran C, Lerner-Ellis J, Xu B, et al. Molecular-based classification algorithm for endometrial carcinoma categorizes ovarian endometrioid carcinoma into prognostically significant groups. Mod Pathol. [Internet] 2017;30(12):1748-1759. Disponible en: https://doi.org/10.1038/modpathol.2017.81
- Shu CA, Zhou Q, Jotwani AR, et al. Ovarian clear cell carcinoma, outcomes by stage: the MSK experience. Gynecol Oncol. [Internet] 2015;139(2):236-41. Disponible en: https://doi.org/10.1016/j.ygyno.2015.09.016
- Nonaka D, Chiriboga L, Soslow RA. Expression of pax8 as a useful marker in distinguishing ovarian carcinomas from mammary carcinomas. Am J Surg Pathol. [Internet] 2008;32(10):1566-71. Disponible en: https://doi.org/10.1097/PAS.0b013e31816d71ad
- Rekhi B, Deodhar KK, Menon S, et al. Napsin A and WT 1 are useful immunohistochemical markers for differentiating clear cell carcinoma ovary from high-grade serous carcinoma. Apmis. [Internet] 2018;126(1):45-55. Disponible en: https://doi.org/10.1111/apm.12784
- Oda K, Hamanishi J, Matsuo K, Hasegawa K. Genomics to immunotherapy of ovarian clear cell carcinoma: Unique opportunities for management. Gynecol Oncol. [Internet] 2018;151(2):381-389. Disponible en: https://doi.org/10.1016/j.ygyno.2018.09.001
- Iida Y, Okamoto A, Hollis RL, Gourley C, Herrington CS. Clear cell carcinoma of the ovary: a clinical and molecular perspective. Int J Gynecol Cancer. [Internet] 2021;31(4):605-616. Disponible en: https://doi.org/10.1136/ijgc-2020-001656
- Slomovitz B, Gourley C, Carey MS, et al. Low-grade serous ovarian cancer: State of the science. Gynecol Oncol. [Internet] 2020;156(3):715-725. Disponible en: https://doi.org/10.1016/j.ygyno.2019.12.033
- Babaier A, Mal H, Alselwi W, Ghatage P. Low-Grade Serous Carcinoma of the Ovary: The Current Status. Diagnostics (Basel). [Internet] 2022;12(2)Disponible en: https://doi.org/10.3390/diagnostics12020458
- Hunter SM, Anglesio MS, Ryland GL, et al. Molecular profiling of low grade serous ovarian tumours identifies novel candidate driver genes. Oncotarget. [Internet] 2015;6(35):37663-77. Disponible en: https://doi.org/10.18632/oncotarget.5438
- ElNaggar A, Robins D, Baca Y, et al. Genomic profiling in low grade serous ovarian cancer: Identification of novel markers for disease diagnosis and therapy. Gynecol Oncol. [Internet] 2022;167(2):306-313. Disponible en: https://doi.org/10.1016/j.ygyno.2022.09.022
- Brown J, Frumovitz M. Mucinous tumors of the ovary: current thoughts on diagnosis and management. Curr Oncol Rep. [Internet] 2014;16(6):389. Disponible en: https://doi.org/10.1007/s11912-014-0389-x
- Morice P, Gouy S, Leary A. Mucinous Ovarian Carcinoma. N Engl J Med. [Internet] 2019;380(13):1256-1266. Disponible en: https://doi.org/10.1056/NEJMra1813254
- Cheasley D, Wakefield MJ, Ryland GL, et al. The molecular origin and taxonomy of mucinous ovarian carcinoma. Nat Commun. [Internet] 2019;10(1):3935. Disponible en: https://doi.org/10.1038/s41467-019-11862-x
- Han R, Madariaga A, Gonzalez-Ochoa E, et al. HER2-low and Overexpression in Mucinous Ovarian Cancer: Analysis of ASCO/CAP and ToGA Immunohistochemical Scoring. Int J Gynecol Pathol. [Internet] 2024;43(3):275-283. Disponible en: https://doi.org/10.1097/pgp.0000000000000972
- Grisham RN, Manning-Geist BL, Chui MH. The highs and lows of serous ovarian cancer. Cancer. [Internet] 2023;129(17):2613-2620. Disponible en: https://doi.org/10.1002/cncr.34903
- Kotnik EN, Mullen MM, Spies NC, et al. Genetic characterization of primary and metastatic high-grade serous ovarian cancer tumors reveals distinct features associated with survival. Commun Biol. [Internet] 2023;6(1):688. Disponible en: https://doi.org/10.1038/s42003-023-05026-3
- Hollis RL, Meynert AM, Michie CO, et al. Multiomic Characterization of High-Grade Serous Ovarian Carcinoma Enables High-Resolution Patient Stratification. Clin Cancer Res. [Internet] 2022;28(16):3546-3556. Disponible en: https://doi.org/10.1158/1078-0432.ccr-22-0368
- Le Saux O, McNeish I, D'Incalci M, Narducci F, Ray-Coquard I. Controversies in the management of serous tubal intra-epithelial carcinoma lesions of the fallopian tube. Int J Gynecol Cancer. [Internet] 2025;35(3):101667. Disponible en: https://doi.org/10.1016/j.ijgc.2025.101667
- Schoutrop E, Moyano-Galceran L, Lheureux S, et al. Molecular, cellular and systemic aspects of epithelial ovarian cancer and its tumor microenvironment. Semin Cancer Biol. [Internet] 2022;86(Pt 3):207-223. Disponible en: https://doi.org/10.1016/j.semcancer.2022.03.027
- Piek JM, van Diest PJ, Zweemer RP, et al. Dysplastic changes in prophylactically removed Fallopian tubes of women predisposed to developing ovarian cancer. J Pathol. [Internet] 2001;195(4):451-6. Disponible en: https://doi.org/10.1002/path.1000
- Wu RC, Wang P, Lin SF, et al. Genomic landscape and evolutionary trajectories of ovarian cancer precursor lesions. J Pathol. [Internet] 2019;248(1):41-50. Disponible en: https://doi.org/10.1002/path.5219
- Shih IM, Wang Y, Wang TL. The Origin of Ovarian Cancer Species and Precancerous Landscape. Am J Pathol. [Internet] 2021;191(1):26-39. Disponible en: https://doi.org/10.1016/j.ajpath.2020.09.006
- Cole AJ, Dwight T, Gill AJ, et al. Assessing mutant p53 in primary high-grade serous ovarian cancer using immunohistochemistry and massively parallel sequencing. Sci Rep. [Internet] 2016;6:26191. Disponible en: https://doi.org/10.1038/srep26191
- Ahmed AA, Etemadmoghadam D, Temple J, et al. Driver mutations in TP53 are ubiquitous in high grade serous carcinoma of the ovary. J Pathol. [Internet] 2010;221(1):49-56. Disponible en: https://doi.org/10.1002/path.2696
- Park E, Han H, Choi SE, et al. p53 Immunohistochemistry and Mutation Types Mismatching in High-Grade Serous Ovarian Cancer. Diagnostics (Basel). [Internet] 2022;12(3)Disponible en: https://doi.org/10.3390/diagnostics12030579
- Neff RT, Senter L, Salani R. BRCA mutation in ovarian cancer: testing, implications and treatment considerations. Ther Adv Med Oncol. [Internet] 2017;9(8):519-531. Disponible en: https://doi.org/10.1177/1758834017714993
- Vlessis K, Purington N, Chun N, Haraldsdottir S, Ford JM. Germline Testing for Patients With BRCA1/2 Mutations on Somatic Tumor Testing. JNCI Cancer Spectr. [Internet] 2020;4(1):pkz095. Disponible en: https://doi.org/10.1093/jncics/pkz095
- Kostov S, Watrowski R, Kornovski Y, et al. Hereditary Gynecologic Cancer Syndromes - A Narrative Review. Onco Targets Ther. [Internet] 2022;15:381-405. Disponible en: https://doi.org/10.2147/ott.s353054
- D'Angelo E, Espinosa I, Felicioni L, Buttitta F, Prat J. Ovarian high-grade serous carcinoma with transitional-like (SET) morphology: a homologous recombination-deficient tumor. Hum Pathol. [Internet] 2023;141:15-21. Disponible en: https://doi.org/10.1016/j.humpath.2023.08.010
- Yang D, Khan S, Sun Y, et al. Association of BRCA1 and BRCA2 mutations with survival, chemotherapy sensitivity, and gene mutator phenotype in patients with ovarian cancer. Jama. [Internet] 2011;306(14):1557-65. Disponible en: https://doi.org/10.1001/jama.2011.1456
- Konstantinopoulos PA, Ceccaldi R, Shapiro GI, D'Andrea AD. Homologous Recombination Deficiency: Exploiting the Fundamental Vulnerability of Ovarian Cancer. Cancer Discov. [Internet] 2015;5(11):1137-54. Disponible en: https://doi.org/10.1158/2159-8290.cd-15-0714
- Ceccaldi R, Rondinelli B, D'Andrea AD. Repair Pathway Choices and Consequences at the Double-Strand Break. Trends Cell Biol. [Internet] 2016;26(1):52-64. Disponible en: https://doi.org/10.1016/j.tcb.2015.07.009
- O'Neil NJ, Bailey ML, Hieter P. Synthetic lethality and cancer. Nat Rev Genet. [Internet] 2017;18(10):613-623. Disponible en: https://doi.org/10.1038/nrg.2017.47
- Abkevich V, Timms KM, Hennessy BT, et al. Patterns of genomic loss of heterozygosity predict homologous recombination repair defects in epithelial ovarian cancer. Br J Cancer. [Internet] 2012;107(10):1776-82. Disponible en: https://doi.org/10.1038/bjc.2012.451
- Feng Z, Zhu C, Zhang X, et al. Comprehensive evaluation of genomic and functional assays for homologous recombination deficiency with high-grade epithelial ovarian cancer: Platinum sensitivity and prognosis. Int J Gynecol Cancer. [Internet] 2025;35(1):100031. Disponible en: https://doi.org/10.1016/j.ijgc.2024.100031
- Kalli KR, Oberg AL, Keeney GL, et al. Folate receptor alpha as a tumor target in epithelial ovarian cancer. Gynecol Oncol. [Internet] 2008;108(3):619-26. Disponible en: https://doi.org/10.1016/j.ygyno.2007.11.020
- Gilbert L, Oaknin A, Matulonis UA, et al. Safety and efficacy of mirvetuximab soravtansine, a folate receptor alpha (FRα)-targeting antibody-drug conjugate (ADC), in combination with bevacizumab in patients with platinum-resistant ovarian cancer. Gynecol Oncol. [Internet] 2023;170:241-247. Disponible en: https://doi.org/10.1016/j.ygyno.2023.01.020
- Tanyi JL, Randall LM, Chambers SK, et al. A Phase III Study of Pafolacianine Injection (OTL38) for Intraoperative Imaging of Folate Receptor-Positive Ovarian Cancer (Study 006). J Clin Oncol. [Internet] 2023;41(2):276-284. Disponible en: https://doi.org/10.1200/jco.22.00291
- Rodriguez GM, Galpin KJC, McCloskey CW, Vanderhyden BC. The Tumor Microenvironment of Epithelial Ovarian Cancer and Its Influence on Response to Immunotherapy. Cancers (Basel). [Internet] 2018;10(8). Disponible en: https://doi.org/10.3390/cancers10080242
- Wang Y, Zhu N, Liu J, et al. Role of tumor microenvironment in ovarian cancer metastasis and clinical advancements. J Transl Med. [Internet] 2025;23(1):539. Disponible en: https://doi.org/10.1186/s12967-025-06508-0
- The Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature. [Internet] 2011;474(7353):609-15. Disponible en: https://doi.org/10.1038/nature10166
- Burger RA, Brady MF, Bookman MA, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. [Internet] 2011;365(26):2473-83. Disponible en: https://doi.org/10.1056/NEJMoa1104390
- Perren TJ, Swart AM, Pfisterer J, et al. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med. [Internet] 2011;365(26):2484-96. Disponible en: https://doi.org/10.1056/NEJMoa1103799
- Oza AM, Cook AD, Pfisterer J, et al. Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): overall survival results of a phase 3 randomised trial. Lancet Oncol. [Internet] 2015;16(8):928-36. Disponible en: https://doi.org/10.1016/s1470-2045(15)00086-8
- Pfisterer J, Joly F, Kristensen G, et al. Optimal Treatment Duration of Bevacizumab as Front-Line Therapy for Advanced Ovarian Cancer: AGO-OVAR 17 BOOST/GINECO OV118/ENGOT Ov-15 Open-Label Randomized Phase III Trial. J Clin Oncol. [Internet] 2023;41(4):893-902. Disponible en: https://doi.org/10.1200/jco.22.01010
- Jin C, Yuan M, Bu H. Antiangiogenic Strategies in Epithelial Ovarian Cancer: Mechanism, Resistance, and Combination Therapy. J Oncol. [Internet] 2022;2022:4880355. Disponible en: https://doi.org/10.1155/2022/4880355
- Moore K, Colombo N, Scambia G, et al. Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N Engl J Med. [Internet] 2018;379(26):2495-2505. Disponible en: https://doi.org/10.1056/NEJMoa1810858
- DiSilvestro P, Banerjee S, Colombo N, et al. Overall Survival With Maintenance Olaparib at a 7-Year Follow-Up in Patients With Newly Diagnosed Advanced Ovarian Cancer and a BRCA Mutation: The SOLO1/GOG 3004 Trial. J Clin Oncol. [Internet] 2023;41(3):609-617. Disponible en: https://doi.org/10.1200/jco.22.01549
- González-Martín A, Pothuri B, Vergote I, et al. Niraparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N Engl J Med. [Internet] 2019;381(25):2391-2402. Disponible en: https://doi.org/10.1056/NEJMoa1910962
- Monk BJ, Barretina-Ginesta MP, Pothuri B, et al. Niraparib first-line maintenance therapy in patients with newly diagnosed advanced ovarian cancer: final overall survival results from the PRIMA/ENGOT-OV26/GOG-3012 trial. Ann Oncol. [Internet] 2024;35(11):981-992. Disponible en: https://doi.org/10.1016/j.annonc.2024.08.2241
- Ray-Coquard I, Pautier P, Pignata S, et al. Olaparib plus Bevacizumab as First-Line Maintenance in Ovarian Cancer. N Engl J Med. [Internet] 2019;381(25):2416-2428. Disponible en: https://doi.org/10.1056/NEJMoa1911361
- González-Martín A, Harter P, Leary A, et al. Newly diagnosed and relapsed epithelial ovarian cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. [Internet] 2023;34(10):833-848. Disponible en: https://doi.org/10.1016/j.annonc.2023.07.011
- Gershenson DM, Sun CC, Westin SN, et al. The genomic landscape of low-grade serous ovarian/peritoneal carcinoma and its impact on clinical outcomes. Gynecol Oncol. [Internet] 2022;165(3):560-567. Disponible en: https://doi.org/10.1016/j.ygyno.2021.11.019
- Farley J, Brady WE, Vathipadiekal V, et al. Selumetinib in women with recurrent low-grade serous carcinoma of the ovary or peritoneum: an open-label, single-arm, phase 2 study. Lancet Oncol. [Internet] 2013;14(2):134-40. Disponible en: https://doi.org/10.1016/s1470-2045(12)70572-7
- Gershenson DM, Miller A, Brady WE, et al. Trametinib versus standard of care in patients with recurrent low-grade serous ovarian cancer (GOG 281/LOGS): an international, randomised, open-label, multicentre, phase 2/3 trial. Lancet. [Internet] 2022;399(10324):541-553. Disponible en: https://doi.org/10.1016/s0140-6736(21)02175-9
- Matulonis UA, Shapira-Frommer R, Santin AD, et al. Antitumor activity and safety of pembrolizumab in patients with advanced recurrent ovarian cancer: results from the phase II KEYNOTE-100 study. Ann Oncol. [Internet] 2019;30(7):1080-1087. Disponible en: https://doi.org/10.1093/annonc/mdz135
- Meric-Bernstam F, Makker V, Oaknin A, et al. Efficacy and Safety of Trastuzumab Deruxtecan in Patients With HER2-Expressing Solid Tumors: Primary Results From the DESTINY-PanTumor02 Phase II Trial. J Clin Oncol. [Internet] 2024;42(1):47-58. Disponible en: https://doi.org/10.1200/jco.23.02005
- McAlpine JN, Wiegand KC, Vang R, et al. HER2 overexpression and amplification is present in a subset of ovarian mucinous carcinomas and can be targeted with trastuzumab therapy. BMC Cancer. [Internet] 2009;9:433. Disponible en: https://doi.org/10.1186/1471-2407-9-433
- Laude É, Azaïs H, Ben Sassi M, Bats AS, Taly V, Laurent-Puig P. Clinical value of circulating tumor DNA for patients with epithelial ovarian cancer. Int J Gynecol Cancer. [Internet] 2025;35(7):101925. Disponible en: https://doi.org/10.1016/j.ijgc.2025.101925
- Zhu JW, Charkhchi P, Akbari MR. Potential clinical utility of liquid biopsies in ovarian cancer. Mol Cancer. [Internet] 2022;21(1):114. Disponible en: https://doi.org/10.1186/s12943-022-01588-8
- Wang B, Yu L, Yang GZ, Luo X, Huang L. Application of multiplex nested methylated specific PCR in early diagnosis of epithelial ovarian cancer. Asian Pac J Cancer Prev. [Internet] 2015;16(7):3003-7. Disponible en: https://doi.org/10.7314/apjcp.2015.16.7.3003
- Zhang X, Li H, Yu X, et al. Analysis of Circulating Tumor Cells in Ovarian Cancer and Their Clinical Value as a Biomarker. Cell Physiol Biochem. [Internet] 2018;48(5):1983-1994. Disponible en: https://doi.org/10.1159/000492521
- Lin KK, Harrell MI, Oza AM, et al. BRCA Reversion Mutations in Circulating Tumor DNA Predict Primary and Acquired Resistance to the PARP Inhibitor Rucaparib in High-Grade Ovarian Carcinoma. Cancer Discov. [Internet] 2019;9(2):210-219. Disponible en: https://doi.org/10.1158/2159-8290.cd-18-0715
- Zhu JW, Wong F, Szymiczek A, et al. Evaluating the Utility of ctDNA in Detecting Residual Cancer and Predicting Recurrence in Patients with Serous Ovarian Cancer. Int J Mol Sci. [Internet] 2023;24(18)Disponible en: https://doi.org/10.3390/ijms241814388
- Chao A, Chen SJ, Chen HC, et al. Mutations in circulating tumor DNA detected in the postoperative period predict poor survival in patients with ovarian cancer. Biomed J. [Internet] 2023;46(5):100563. Disponible en: https://doi.org/10.1016/j.bj.2022.09.004
- Heo J, Kim YN, Shin S, et al. Serial Circulating Tumor DNA Analysis with a Tumor-Naïve Next-Generation Sequencing Panel Detects Minimal Residual Disease and Predicts Outcome in Ovarian Cancer. Cancer Res. [Internet] 2024;84(3):468-478. Disponible en: https://doi.org/10.1158/0008-5472.can-23-1429

















