Molecular biology of epithelial ovarian cancer
Biología molecular del cáncer epitelial de ovario
How to Cite
Download Citation

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Show authors biography
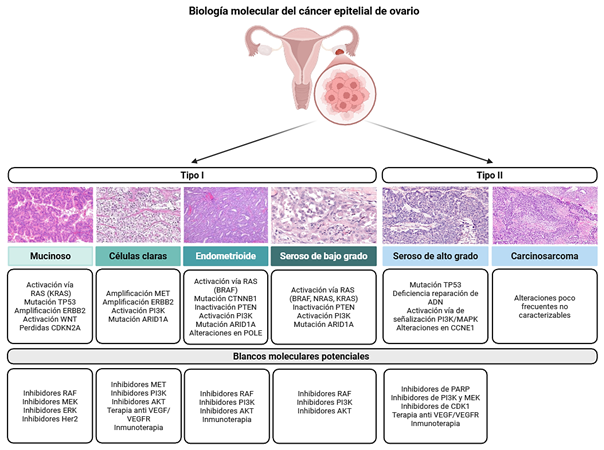
Introduction: epithelial ovarian cancer is a heterogeneous neoplasm with high mortality, characterized by diverse histological and molecular subtypes that determine its clinical behavior, prognosis, and therapeutic response. High-grade serous carcinoma is the most frequent subtype and is associated with diagnosis at advanced stages and a high recurrence rate.
Methods: a narrative review of the scientific literature was conducted focusing on the histopathological, molecular, and therapeutic aspects of epithelial ovarian cancer, with particular emphasis on molecular characterization, advances in targeted therapies, and the emerging role of liquid biopsy.
Results: high-grade serous carcinoma exhibits marked genomic instability, ubiquitous TP53 mutations, and alterations in the homologous recombination repair pathway, particularly germline or somatic mutations in BRCA1/2. These findings have enabled the incorporation of PARP inhibitors as a therapeutic strategy. Other less frequent subtypes—endometrioid, clear cell, mucinous, and low-grade serous carcinomas—display distinct molecular profiles, with recurrent mutations in ARID1A, PIK3CA, KRAS, or CTNNB1, which carry specific prognostic and therapeutic implications. Transcriptomic classification proposed by The Cancer Genome Atlas (TCGA) identifies immunoreactive, mesenchymal, proliferative, and differentiated subtypes, reflecting the biological complexity of the tumor. In addition, maintenance strategies with targeted therapies, such as bevacizumab and PARP inhibitors (olaparib, niraparib, and rucaparib), have demonstrated improved outcomes in selected patients. Liquid biopsy is emerging as a promising tool for dynamic disease monitoring and early detection of recurrence.
Conclusions: integration of clinical, molecular, and tumor microenvironment information is essential to advance toward truly personalized management of epithelial ovarian cancer, optimizing therapeutic selection and improving clinical outcomes.
Article visits 0 | PDF visits 0
Downloads
- Bray F, Laversanne M, Sung H, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. [Internet] 2024;74(3):229-263. Disponible en: https://doi.org/10.3322/caac.21834
- Wang M, Bi Y, Jin Y, Zheng ZJ. Global Incidence of Ovarian Cancer According to Histologic Subtype: A Population-Based Cancer Registry Study. JCO Glob Oncol. [Internet] 2024;10:e2300393. Disponible en: https://doi.org/10.1200/go.23.00393
- Rojas V, Hirshfield KM, Ganesan S, Rodriguez-Rodriguez L. Molecular Characterization of Epithelial Ovarian Cancer: Implications for Diagnosis and Treatment. Int J Mol Sci. [Internet] 2016;17(12). Disponible en: https://doi.org/10.3390/ijms17122113
- Soovares P, Pasanen A, Similä-Maarala J, Bützow R, Lassus H. Clinical factors and biomarker profiles associated with patient outcome in endometrioid ovarian carcinoma - Emphasis on tumor grade. Gynecol Oncol. [Internet] 2022;164(1):187-194. Disponible en: https://doi.org/10.1016/j.ygyno.2021.10.078
- Köbel M, Rahimi K, Rambau PF, et al. An Immunohistochemical Algorithm for Ovarian Carcinoma Typing. Int J Gynecol Pathol. [Internet] 2016;35(5):430-41. Disponible en: https://doi.org/10.1097/pgp.0000000000000274
- Sieh W, Köbel M, Longacre TA, et al. Hormone-receptor expression and ovarian cancer survival: an Ovarian Tumor Tissue Analysis consortium study. Lancet Oncol. [Internet] 2013;14(9):853-62. Disponible en: https://doi.org/10.1016/s1470-2045(13)70253-5
- McCluggage WG. Morphological subtypes of ovarian carcinoma: a review with emphasis on new developments and pathogenesis. Pathology. [Internet] 2011;43(5):420-32. Disponible en: https://doi.org/10.1097/PAT.0b013e328348a6e7
- Hollis RL, Thomson JP, Stanley B, et al. Molecular stratification of endometrioid ovarian carcinoma predicts clinical outcome. Nat Commun. [Internet] 2020;11(1):4995. Disponible en: https://doi.org/10.1038/s41467-020-18819-5
- Parra-Herran C, Lerner-Ellis J, Xu B, et al. Molecular-based classification algorithm for endometrial carcinoma categorizes ovarian endometrioid carcinoma into prognostically significant groups. Mod Pathol. [Internet] 2017;30(12):1748-1759. Disponible en: https://doi.org/10.1038/modpathol.2017.81
- Shu CA, Zhou Q, Jotwani AR, et al. Ovarian clear cell carcinoma, outcomes by stage: the MSK experience. Gynecol Oncol. [Internet] 2015;139(2):236-41. Disponible en: https://doi.org/10.1016/j.ygyno.2015.09.016
- Nonaka D, Chiriboga L, Soslow RA. Expression of pax8 as a useful marker in distinguishing ovarian carcinomas from mammary carcinomas. Am J Surg Pathol. [Internet] 2008;32(10):1566-71. Disponible en: https://doi.org/10.1097/PAS.0b013e31816d71ad
- Rekhi B, Deodhar KK, Menon S, et al. Napsin A and WT 1 are useful immunohistochemical markers for differentiating clear cell carcinoma ovary from high-grade serous carcinoma. Apmis. [Internet] 2018;126(1):45-55. Disponible en: https://doi.org/10.1111/apm.12784
- Oda K, Hamanishi J, Matsuo K, Hasegawa K. Genomics to immunotherapy of ovarian clear cell carcinoma: Unique opportunities for management. Gynecol Oncol. [Internet] 2018;151(2):381-389. Disponible en: https://doi.org/10.1016/j.ygyno.2018.09.001
- Iida Y, Okamoto A, Hollis RL, Gourley C, Herrington CS. Clear cell carcinoma of the ovary: a clinical and molecular perspective. Int J Gynecol Cancer. [Internet] 2021;31(4):605-616. Disponible en: https://doi.org/10.1136/ijgc-2020-001656
- Slomovitz B, Gourley C, Carey MS, et al. Low-grade serous ovarian cancer: State of the science. Gynecol Oncol. [Internet] 2020;156(3):715-725. Disponible en: https://doi.org/10.1016/j.ygyno.2019.12.033
- Babaier A, Mal H, Alselwi W, Ghatage P. Low-Grade Serous Carcinoma of the Ovary: The Current Status. Diagnostics (Basel). [Internet] 2022;12(2)Disponible en: https://doi.org/10.3390/diagnostics12020458
- Hunter SM, Anglesio MS, Ryland GL, et al. Molecular profiling of low grade serous ovarian tumours identifies novel candidate driver genes. Oncotarget. [Internet] 2015;6(35):37663-77. Disponible en: https://doi.org/10.18632/oncotarget.5438
- ElNaggar A, Robins D, Baca Y, et al. Genomic profiling in low grade serous ovarian cancer: Identification of novel markers for disease diagnosis and therapy. Gynecol Oncol. [Internet] 2022;167(2):306-313. Disponible en: https://doi.org/10.1016/j.ygyno.2022.09.022
- Brown J, Frumovitz M. Mucinous tumors of the ovary: current thoughts on diagnosis and management. Curr Oncol Rep. [Internet] 2014;16(6):389. Disponible en: https://doi.org/10.1007/s11912-014-0389-x
- Morice P, Gouy S, Leary A. Mucinous Ovarian Carcinoma. N Engl J Med. [Internet] 2019;380(13):1256-1266. Disponible en: https://doi.org/10.1056/NEJMra1813254
- Cheasley D, Wakefield MJ, Ryland GL, et al. The molecular origin and taxonomy of mucinous ovarian carcinoma. Nat Commun. [Internet] 2019;10(1):3935. Disponible en: https://doi.org/10.1038/s41467-019-11862-x
- Han R, Madariaga A, Gonzalez-Ochoa E, et al. HER2-low and Overexpression in Mucinous Ovarian Cancer: Analysis of ASCO/CAP and ToGA Immunohistochemical Scoring. Int J Gynecol Pathol. [Internet] 2024;43(3):275-283. Disponible en: https://doi.org/10.1097/pgp.0000000000000972
- Grisham RN, Manning-Geist BL, Chui MH. The highs and lows of serous ovarian cancer. Cancer. [Internet] 2023;129(17):2613-2620. Disponible en: https://doi.org/10.1002/cncr.34903
- Kotnik EN, Mullen MM, Spies NC, et al. Genetic characterization of primary and metastatic high-grade serous ovarian cancer tumors reveals distinct features associated with survival. Commun Biol. [Internet] 2023;6(1):688. Disponible en: https://doi.org/10.1038/s42003-023-05026-3
- Hollis RL, Meynert AM, Michie CO, et al. Multiomic Characterization of High-Grade Serous Ovarian Carcinoma Enables High-Resolution Patient Stratification. Clin Cancer Res. [Internet] 2022;28(16):3546-3556. Disponible en: https://doi.org/10.1158/1078-0432.ccr-22-0368
- Le Saux O, McNeish I, D'Incalci M, Narducci F, Ray-Coquard I. Controversies in the management of serous tubal intra-epithelial carcinoma lesions of the fallopian tube. Int J Gynecol Cancer. [Internet] 2025;35(3):101667. Disponible en: https://doi.org/10.1016/j.ijgc.2025.101667
- Schoutrop E, Moyano-Galceran L, Lheureux S, et al. Molecular, cellular and systemic aspects of epithelial ovarian cancer and its tumor microenvironment. Semin Cancer Biol. [Internet] 2022;86(Pt 3):207-223. Disponible en: https://doi.org/10.1016/j.semcancer.2022.03.027
- Piek JM, van Diest PJ, Zweemer RP, et al. Dysplastic changes in prophylactically removed Fallopian tubes of women predisposed to developing ovarian cancer. J Pathol. [Internet] 2001;195(4):451-6. Disponible en: https://doi.org/10.1002/path.1000
- Wu RC, Wang P, Lin SF, et al. Genomic landscape and evolutionary trajectories of ovarian cancer precursor lesions. J Pathol. [Internet] 2019;248(1):41-50. Disponible en: https://doi.org/10.1002/path.5219
- Shih IM, Wang Y, Wang TL. The Origin of Ovarian Cancer Species and Precancerous Landscape. Am J Pathol. [Internet] 2021;191(1):26-39. Disponible en: https://doi.org/10.1016/j.ajpath.2020.09.006
- Cole AJ, Dwight T, Gill AJ, et al. Assessing mutant p53 in primary high-grade serous ovarian cancer using immunohistochemistry and massively parallel sequencing. Sci Rep. [Internet] 2016;6:26191. Disponible en: https://doi.org/10.1038/srep26191
- Ahmed AA, Etemadmoghadam D, Temple J, et al. Driver mutations in TP53 are ubiquitous in high grade serous carcinoma of the ovary. J Pathol. [Internet] 2010;221(1):49-56. Disponible en: https://doi.org/10.1002/path.2696
- Park E, Han H, Choi SE, et al. p53 Immunohistochemistry and Mutation Types Mismatching in High-Grade Serous Ovarian Cancer. Diagnostics (Basel). [Internet] 2022;12(3)Disponible en: https://doi.org/10.3390/diagnostics12030579
- Neff RT, Senter L, Salani R. BRCA mutation in ovarian cancer: testing, implications and treatment considerations. Ther Adv Med Oncol. [Internet] 2017;9(8):519-531. Disponible en: https://doi.org/10.1177/1758834017714993
- Vlessis K, Purington N, Chun N, Haraldsdottir S, Ford JM. Germline Testing for Patients With BRCA1/2 Mutations on Somatic Tumor Testing. JNCI Cancer Spectr. [Internet] 2020;4(1):pkz095. Disponible en: https://doi.org/10.1093/jncics/pkz095
- Kostov S, Watrowski R, Kornovski Y, et al. Hereditary Gynecologic Cancer Syndromes - A Narrative Review. Onco Targets Ther. [Internet] 2022;15:381-405. Disponible en: https://doi.org/10.2147/ott.s353054
- D'Angelo E, Espinosa I, Felicioni L, Buttitta F, Prat J. Ovarian high-grade serous carcinoma with transitional-like (SET) morphology: a homologous recombination-deficient tumor. Hum Pathol. [Internet] 2023;141:15-21. Disponible en: https://doi.org/10.1016/j.humpath.2023.08.010
- Yang D, Khan S, Sun Y, et al. Association of BRCA1 and BRCA2 mutations with survival, chemotherapy sensitivity, and gene mutator phenotype in patients with ovarian cancer. Jama. [Internet] 2011;306(14):1557-65. Disponible en: https://doi.org/10.1001/jama.2011.1456
- Konstantinopoulos PA, Ceccaldi R, Shapiro GI, D'Andrea AD. Homologous Recombination Deficiency: Exploiting the Fundamental Vulnerability of Ovarian Cancer. Cancer Discov. [Internet] 2015;5(11):1137-54. Disponible en: https://doi.org/10.1158/2159-8290.cd-15-0714
- Ceccaldi R, Rondinelli B, D'Andrea AD. Repair Pathway Choices and Consequences at the Double-Strand Break. Trends Cell Biol. [Internet] 2016;26(1):52-64. Disponible en: https://doi.org/10.1016/j.tcb.2015.07.009
- O'Neil NJ, Bailey ML, Hieter P. Synthetic lethality and cancer. Nat Rev Genet. [Internet] 2017;18(10):613-623. Disponible en: https://doi.org/10.1038/nrg.2017.47
- Abkevich V, Timms KM, Hennessy BT, et al. Patterns of genomic loss of heterozygosity predict homologous recombination repair defects in epithelial ovarian cancer. Br J Cancer. [Internet] 2012;107(10):1776-82. Disponible en: https://doi.org/10.1038/bjc.2012.451
- Feng Z, Zhu C, Zhang X, et al. Comprehensive evaluation of genomic and functional assays for homologous recombination deficiency with high-grade epithelial ovarian cancer: Platinum sensitivity and prognosis. Int J Gynecol Cancer. [Internet] 2025;35(1):100031. Disponible en: https://doi.org/10.1016/j.ijgc.2024.100031
- Kalli KR, Oberg AL, Keeney GL, et al. Folate receptor alpha as a tumor target in epithelial ovarian cancer. Gynecol Oncol. [Internet] 2008;108(3):619-26. Disponible en: https://doi.org/10.1016/j.ygyno.2007.11.020
- Gilbert L, Oaknin A, Matulonis UA, et al. Safety and efficacy of mirvetuximab soravtansine, a folate receptor alpha (FRα)-targeting antibody-drug conjugate (ADC), in combination with bevacizumab in patients with platinum-resistant ovarian cancer. Gynecol Oncol. [Internet] 2023;170:241-247. Disponible en: https://doi.org/10.1016/j.ygyno.2023.01.020
- Tanyi JL, Randall LM, Chambers SK, et al. A Phase III Study of Pafolacianine Injection (OTL38) for Intraoperative Imaging of Folate Receptor-Positive Ovarian Cancer (Study 006). J Clin Oncol. [Internet] 2023;41(2):276-284. Disponible en: https://doi.org/10.1200/jco.22.00291
- Rodriguez GM, Galpin KJC, McCloskey CW, Vanderhyden BC. The Tumor Microenvironment of Epithelial Ovarian Cancer and Its Influence on Response to Immunotherapy. Cancers (Basel). [Internet] 2018;10(8). Disponible en: https://doi.org/10.3390/cancers10080242
- Wang Y, Zhu N, Liu J, et al. Role of tumor microenvironment in ovarian cancer metastasis and clinical advancements. J Transl Med. [Internet] 2025;23(1):539. Disponible en: https://doi.org/10.1186/s12967-025-06508-0
- The Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature. [Internet] 2011;474(7353):609-15. Disponible en: https://doi.org/10.1038/nature10166
- Burger RA, Brady MF, Bookman MA, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. [Internet] 2011;365(26):2473-83. Disponible en: https://doi.org/10.1056/NEJMoa1104390
- Perren TJ, Swart AM, Pfisterer J, et al. A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med. [Internet] 2011;365(26):2484-96. Disponible en: https://doi.org/10.1056/NEJMoa1103799
- Oza AM, Cook AD, Pfisterer J, et al. Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): overall survival results of a phase 3 randomised trial. Lancet Oncol. [Internet] 2015;16(8):928-36. Disponible en: https://doi.org/10.1016/s1470-2045(15)00086-8
- Pfisterer J, Joly F, Kristensen G, et al. Optimal Treatment Duration of Bevacizumab as Front-Line Therapy for Advanced Ovarian Cancer: AGO-OVAR 17 BOOST/GINECO OV118/ENGOT Ov-15 Open-Label Randomized Phase III Trial. J Clin Oncol. [Internet] 2023;41(4):893-902. Disponible en: https://doi.org/10.1200/jco.22.01010
- Jin C, Yuan M, Bu H. Antiangiogenic Strategies in Epithelial Ovarian Cancer: Mechanism, Resistance, and Combination Therapy. J Oncol. [Internet] 2022;2022:4880355. Disponible en: https://doi.org/10.1155/2022/4880355
- Moore K, Colombo N, Scambia G, et al. Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N Engl J Med. [Internet] 2018;379(26):2495-2505. Disponible en: https://doi.org/10.1056/NEJMoa1810858
- DiSilvestro P, Banerjee S, Colombo N, et al. Overall Survival With Maintenance Olaparib at a 7-Year Follow-Up in Patients With Newly Diagnosed Advanced Ovarian Cancer and a BRCA Mutation: The SOLO1/GOG 3004 Trial. J Clin Oncol. [Internet] 2023;41(3):609-617. Disponible en: https://doi.org/10.1200/jco.22.01549
- González-Martín A, Pothuri B, Vergote I, et al. Niraparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N Engl J Med. [Internet] 2019;381(25):2391-2402. Disponible en: https://doi.org/10.1056/NEJMoa1910962
- Monk BJ, Barretina-Ginesta MP, Pothuri B, et al. Niraparib first-line maintenance therapy in patients with newly diagnosed advanced ovarian cancer: final overall survival results from the PRIMA/ENGOT-OV26/GOG-3012 trial. Ann Oncol. [Internet] 2024;35(11):981-992. Disponible en: https://doi.org/10.1016/j.annonc.2024.08.2241
- Ray-Coquard I, Pautier P, Pignata S, et al. Olaparib plus Bevacizumab as First-Line Maintenance in Ovarian Cancer. N Engl J Med. [Internet] 2019;381(25):2416-2428. Disponible en: https://doi.org/10.1056/NEJMoa1911361
- González-Martín A, Harter P, Leary A, et al. Newly diagnosed and relapsed epithelial ovarian cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. [Internet] 2023;34(10):833-848. Disponible en: https://doi.org/10.1016/j.annonc.2023.07.011
- Gershenson DM, Sun CC, Westin SN, et al. The genomic landscape of low-grade serous ovarian/peritoneal carcinoma and its impact on clinical outcomes. Gynecol Oncol. [Internet] 2022;165(3):560-567. Disponible en: https://doi.org/10.1016/j.ygyno.2021.11.019
- Farley J, Brady WE, Vathipadiekal V, et al. Selumetinib in women with recurrent low-grade serous carcinoma of the ovary or peritoneum: an open-label, single-arm, phase 2 study. Lancet Oncol. [Internet] 2013;14(2):134-40. Disponible en: https://doi.org/10.1016/s1470-2045(12)70572-7
- Gershenson DM, Miller A, Brady WE, et al. Trametinib versus standard of care in patients with recurrent low-grade serous ovarian cancer (GOG 281/LOGS): an international, randomised, open-label, multicentre, phase 2/3 trial. Lancet. [Internet] 2022;399(10324):541-553. Disponible en: https://doi.org/10.1016/s0140-6736(21)02175-9
- Matulonis UA, Shapira-Frommer R, Santin AD, et al. Antitumor activity and safety of pembrolizumab in patients with advanced recurrent ovarian cancer: results from the phase II KEYNOTE-100 study. Ann Oncol. [Internet] 2019;30(7):1080-1087. Disponible en: https://doi.org/10.1093/annonc/mdz135
- Meric-Bernstam F, Makker V, Oaknin A, et al. Efficacy and Safety of Trastuzumab Deruxtecan in Patients With HER2-Expressing Solid Tumors: Primary Results From the DESTINY-PanTumor02 Phase II Trial. J Clin Oncol. [Internet] 2024;42(1):47-58. Disponible en: https://doi.org/10.1200/jco.23.02005
- McAlpine JN, Wiegand KC, Vang R, et al. HER2 overexpression and amplification is present in a subset of ovarian mucinous carcinomas and can be targeted with trastuzumab therapy. BMC Cancer. [Internet] 2009;9:433. Disponible en: https://doi.org/10.1186/1471-2407-9-433
- Laude É, Azaïs H, Ben Sassi M, Bats AS, Taly V, Laurent-Puig P. Clinical value of circulating tumor DNA for patients with epithelial ovarian cancer. Int J Gynecol Cancer. [Internet] 2025;35(7):101925. Disponible en: https://doi.org/10.1016/j.ijgc.2025.101925
- Zhu JW, Charkhchi P, Akbari MR. Potential clinical utility of liquid biopsies in ovarian cancer. Mol Cancer. [Internet] 2022;21(1):114. Disponible en: https://doi.org/10.1186/s12943-022-01588-8
- Wang B, Yu L, Yang GZ, Luo X, Huang L. Application of multiplex nested methylated specific PCR in early diagnosis of epithelial ovarian cancer. Asian Pac J Cancer Prev. [Internet] 2015;16(7):3003-7. Disponible en: https://doi.org/10.7314/apjcp.2015.16.7.3003
- Zhang X, Li H, Yu X, et al. Analysis of Circulating Tumor Cells in Ovarian Cancer and Their Clinical Value as a Biomarker. Cell Physiol Biochem. [Internet] 2018;48(5):1983-1994. Disponible en: https://doi.org/10.1159/000492521
- Lin KK, Harrell MI, Oza AM, et al. BRCA Reversion Mutations in Circulating Tumor DNA Predict Primary and Acquired Resistance to the PARP Inhibitor Rucaparib in High-Grade Ovarian Carcinoma. Cancer Discov. [Internet] 2019;9(2):210-219. Disponible en: https://doi.org/10.1158/2159-8290.cd-18-0715
- Zhu JW, Wong F, Szymiczek A, et al. Evaluating the Utility of ctDNA in Detecting Residual Cancer and Predicting Recurrence in Patients with Serous Ovarian Cancer. Int J Mol Sci. [Internet] 2023;24(18)Disponible en: https://doi.org/10.3390/ijms241814388
- Chao A, Chen SJ, Chen HC, et al. Mutations in circulating tumor DNA detected in the postoperative period predict poor survival in patients with ovarian cancer. Biomed J. [Internet] 2023;46(5):100563. Disponible en: https://doi.org/10.1016/j.bj.2022.09.004
- Heo J, Kim YN, Shin S, et al. Serial Circulating Tumor DNA Analysis with a Tumor-Naïve Next-Generation Sequencing Panel Detects Minimal Residual Disease and Predicts Outcome in Ovarian Cancer. Cancer Res. [Internet] 2024;84(3):468-478. Disponible en: https://doi.org/10.1158/0008-5472.can-23-1429

















