Biología molecular del cáncer de próstata
Molecular biology of prostate cancer
Cómo citar
Descargar cita

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-SinDerivadas 4.0.
Mostrar biografía de los autores
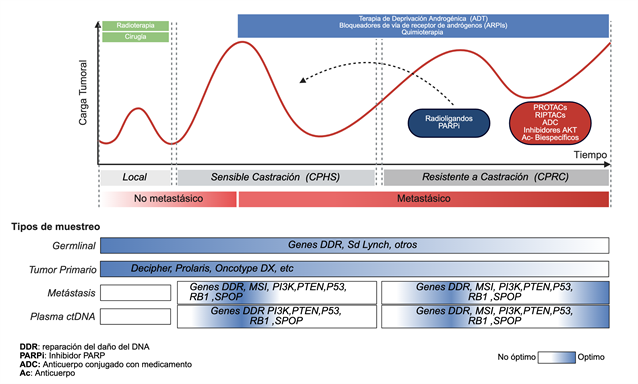
Introducción: el cáncer de próstata (CaP) es la neoplasia maligna más frecuente en hombres y una causa relevante de mortalidad oncológica mundial. Su heterogeneidad, desde tumores indolentes hasta formas agresivas, dificulta la estratificación del riesgo y la selección terapéutica, pero los avances en biología molecular han aclarado las alteraciones genómicas y epigenéticas implicadas en la tumorogénesis, progresión y resistencia, impulsando la medicina de precisión.
Métodos: se realizó una revisión narrativa de la literatura sobre vías moleculares clave, alteraciones genómicas clínicamente relevantes y estrategias terapéuticas emergentes basadas en biomarcadores en CaP localizado y metastásico.
Resultados: las mutaciones germinales y somáticas en genes de reparación del ADN, como BRCA1, BRCA2 y ATM, se relacionan con mayor susceptibilidad, fenotipos agresivos y sensibilidad a inhibidores PARP y esquemas con platino. La pérdida de genes supresores tumorales (PTEN, TP53, RB1) favorece la inestabilidad genómica, la resistencia a la castración y un pronóstico desfavorable. Los clasificadores genómicos (Oncotype DX, Decipher) refinan la estratificación del riesgo y orientan la intensificación o desescalamiento terapéutico en enfermedad localizada, mientras que el testeo genético reflejo y los estudios clínicos dirigidos por biomarcadores ejemplifican la integración clínica de la información molecular. En enfermedad avanzada, terapias dirigidas al receptor androgénico, a la reparación del ADN y al PSMA están transformando el manejo del CaP.
Conclusiones: la caracterización molecular del CaP permite intervenciones guiadas por biomarcadores que optimizan las decisiones terapéuticas y los desenlaces clínicos. La incorporación sistemática del perfil genómico será esencial para consolidar la medicina de precisión en el tratamiento.
Visitas del artículo 0 | Visitas PDF 0
Descargas
- Ferlay J., Ervik M., Lam F., et al. Global Cancer Observatory: Cancer Today (version 1.1) Lyon, France: International Agency for Research on Cancer; [Internet] 2024 Disponible en: https://gco.iarc.who.int/today
- Kwon W-A, Joung YJ. Precision Targeting in Metastatic Prostate Cancer: Molecular Insights to Therapeutic Frontiers. Biomolecules [Internet] 2025;15(5):625. Disponible en: https://doi.org/10.3390/biom15050625
- Pritchard CC, Mateo J, Walsh MF, et al. Inherited DNA-Repair Gene Mutations in Men with Metastatic Prostate Cancer. N Engl J Med [Internet] 2016;375(5):443–53. Disponible en: https://doi.org/10.1056/NEJMoa1603144
- Lotan TL, Wei W, Morais CL, et al. PTEN Loss as Determined by Clinical-grade Immunohistochemistry Assay Is Associated with Worse Recurrence-free Survival in Prostate Cancer. Eur Urol Focus [Internet] 2016;2(2):180–88. Disponible en: https://doi.org/10.1016/j.euf.2015.07.005
- Berchuck JE, Zhang Z, Silver R, et al. Impact of Pathogenic Germline DNA Damage Repair alterations on Response to Intense Neoadjuvant Androgen Deprivation Therapy in High-risk Localized Prostate Cancer. Eur Urol [Internet] 2021;80(3):295–303. Disponible en: https://doi.org/10.1016/j.eururo.2021.03.031
- National Comprehensive Cancer N. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®): Prostate Cancer. Version 1.2025. 2025
- Prendeville S, Kaur H, Ansari S, et al. Somatic Tumor Testing in Prostate Cancer: Experience of a Tertiary Care Center Including Pathologist-Driven Reflex Testing of Localized Tumors at Diagnosis. Mod Pathol [Internet] 2024;37(6):100489. Disponible en: https://doi.org/10.1016/j.modpat.2024.100489
- Parker C, Castro E, Fizazi K, et al. Prostate cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol [Internet] 2020;31(9):1119–34. Disponible en: https://doi.org/10.1016/j.annonc.2020.06.011
- Cornford P, Tilki D, Van Den Bergh RC, et al. EAU-EANM-ESTRO-ESUR-ISUP-SIOG-Guidelines on Prostate Cancer. 2025. [Internet] Disponible en: https://uroweb.org/guidelines/prostate-cancer.
- Tuffaha H, Edmunds K, Fairbairn D, et al. Guidelines for genetic testing in prostate cancer: a scoping review. Prostate Cancer Prostatic Dis [Internet] 2024;27(4):594–603. Disponible en: https://doi.org/10.1038/s41391-023-00676-0
- Knezevic D, Goddard AD, Natraj N, et al. Analytical validation of the Oncotype DX prostate cancer assay - a clinical RT-PCR assay optimized for prostate needle biopsies. BMC Genomics [Internet] 2013;14:690. Disponible en: https://doi.org/10.1186/1471-2164-14-690
- Van den Broeck T, Moris L, Gevaert T, et al. Validation of the Decipher Test for Predicting Distant Metastatic Recurrence in Men with High-risk Nonmetastatic Prostate Cancer 10 Years After Surgery. Eur Urol Oncol [Internet] 2019;2(5):589–96. Disponible en: https://doi.org/10.1016/j.euo.2018.12.007
- Tabriz AA, Boyer MJ, Gordon AM, et al. Impact of Genomic Classifiers on Risk Stratification and Treatment Intensity in Patients With Localized Prostate Cancer : A Systematic Review. Ann Intern Med [Internet] 2025;178(2):218–28. Disponible en: https://doi.org/10.7326/ANNALS-24-00700
- Abdollah F, Dalela D, Sood A, et al. Impact of Adjuvant Radiotherapy in Node-positive Prostate Cancer Patients: The Importance of Patient Selection. Eur Urol [Internet] 2018;74(3):253–56. Disponible en: https://doi.org/10.1016/j.eururo.2018.04.017
- Pedrani M, Salfi G, Merler S, et al. Prognostic and Predictive Role of SPOP Mutations in Prostate Cancer: A Systematic Review and Meta-analysis. Eur Urol Oncol [Internet] 2024;7(6):1199–215. Disponible en: https://doi.org/10.1016/j.euo.2024.04.011
- Boysen G, Barbieri CE, Prandi D, et al. SPOP mutation leads to genomic instability in prostate cancer. Elife [Internet] 2015;4. Disponible en: https://doi.org/10.7554/eLife.09207
- Bancroft EK, Page EC, Castro E, et al. Targeted prostate cancer screening in BRCA1 and BRCA2 mutation carriers: results from the initial screening round of the IMPACT study. Eur Urol [Internet] 2014;66(3):489–99. Disponible en: https://doi.org/10.1016/j.eururo.2014.01.003
- Habaka M, Daly GR, Shinyanbola D, et al. PARP Inhibitors in the Neoadjuvant Setting; A Comprehensive Overview of the Rationale for their Use, Past and Ongoing Clinical Trials. Curr Oncol Rep [Internet] 2025;27(5):533–51. Disponible en: https://doi.org/10.1007/s11912-025-01669-z
- Cheng HH, Callis S, Lin DW, et al. SWOG S2210: A phase II study of neoadjuvant carboplatin for localized, high-risk prostate cancer with germline BRCA1/2 mutations. Journal of Clinical Oncology [Internet] 2024;42(4_suppl):TPS354–TPS54. Disponible en: https://doi.org/: 10.1200/JCO.2024.42.4_suppl.TPS354
- Krafft U, Hadaschik BA, Luckerath K, et al. A New Chapter in Neoadjuvant Therapy for High-risk Prostate Cancer? Eur Urol [Internet] 2024;85(3):227–28. Disponible en: https://doi.org/10.1016/j.eururo.2023.09.022
- Atiq MO, Pastor DM, Karzai F, et al. First-line pembrolizumab plus androgen deprivation therapy for locally advanced microsatellite instability-high prostate cancer in a patient with Muir-Torre syndrome: A case report. Front Oncol [Internet] 2023;13:1126476. Disponible en: https://doi.org/10.3389/fonc.2023.1126476
- Trujillo B, Wu A, Wetterskog D, et al. Blood-based liquid biopsies for prostate cancer: clinical opportunities and challenges. British Journal of Cancer [Internet] 2022;127(8):1394–402. Disponible en: https://doi.org/10.1038/s41416-022-01881-9
- Fujita K, Nonomura N. Role of Androgen Receptor in Prostate Cancer: A Review. World J Mens Health [Internet] 2019;37(3):288–95. Disponible en:
- Han D, Labaf M, Zhao Y, et al. Androgen receptor splice variants drive castration-resistant prostate cancer metastasis by activating distinct transcriptional programs. The Journal of Clinical Investigation [Internet] 2024;134(11). Disponible en: https://doi.org/10.1172/JCI168649
- Moigne RL, Mawji NR, Banuelos CA, et al. Abstract 1292: A new generation of N-terminal domain androgen receptor inhibitors, with improved pharmaceutical properties, in castration-resistant prostate cancer models. Cancer research [Internet] 2019;79(13_Supplement):1292–92. Disponible en: https://doi.org/10.1158/1538-7445.Am2019-1292
- Hung C-L, Liu H-H, Fu C-W, et al. Targeting androgen receptor and the variants by an orally bioavailable Proteolysis Targeting Chimeras compound in castration resistant prostate cancer. EBioMedicine [Internet] 2023;90. Disponible en: https://doi.org/10.1016/j.ebiom.2023.104500
- Grewal K, Dorff BT, Mukhida SS, et al. Advances in Targeted Therapy for Metastatic Prostate Cancer. Current Treatment Options in Oncology [Internet] 2025;26(6):465–75. Disponible en: https://doi.org/10.1007/s11864-025-01323-7
- Wu F, Zhang H, Hao M. Interactions between key genes and pathways in prostate cancer progression and therapy resistance. Frontiers in Oncology [Internet] 2025;Volume 15 - 2025. Disponible en: https://doi.org/10.3389/fonc.2025.1467540
- Chirnomas D, Hornberger KR, Crews CM. Protein degraders enter the clinic — a new approach to cancer therapy. Nature Reviews Clinical Oncology [Internet] 2023;20(4):265–78. Disponible en: https://doi.org/10.1038/s41571-023-00736-3
- Gao X, III HAB, Vuky J, et al. Phase 1/2 study of ARV-110, an androgen receptor (AR) PROTAC degrader, in metastatic castration-resistant prostate cancer (mCRPC). Journal of Clinical Oncology [Internet] 2022;40(6_suppl):17–17.Disponible en: https://doi.org/10.1200/JCO.2022.40.6_suppl.017
- Petrylak DP, McKean M, Lang JM, et al. ARV-766, a proteolysis targeting chimera (PROTAC) androgen receptor (AR) degrader, in metastatic castration-resistant prostate cancer (mCRPC): Initial results of a phase 1/2 study. Journal of Clinical Oncology [Internet] 2024;42(16_suppl):5011–11.Disponible en: https://doi.org/10.1200/JCO.2024.42.16_suppl.5011
- Rathkopf DE, Patel MR, Choudhury AD, et al. Safety and clinical activity of BMS-986365 (CC-94676), a dual androgen receptor ligand-directed degrader and antagonist, in heavily pretreated patients with metastatic castration-resistant prostate cancer. Annals of Oncology [Internet] 2025;36(1):76–88.Disponible en: https://doi.org/10.1016/j.annonc.2024.09.005
- Fizazi K, Bernard-Tessier A, Roubaud G, et al. Targeted Inhibition of CYP11A1 in Castration-Resistant Prostate Cancer. NEJM Evid [Internet] 2024;3(1):EVIDoa2300171. Disponible en: https://doi.org/10.1056/EVIDoa2300171
- Raina K, Eastman KJ, Yu X, et al. An oral androgen receptor RIPTAC for prostate cancer. Journal of Clinical Oncology [Internet] 2023;41(6_suppl):184–84.Disponible en:https://doi.org/10.1200/JCO.2023.41.6_suppl.184
- Bourlon MT, Valdez P, Castro E. Development of PARP inhibitors in advanced prostate cancer. Therapeutic Advances in Medical Oncology [Internet] 2024;16. Disponible en: https://doi.org/10.1177/17588359231221337
- Polkinghorn WR, Parker JS, Lee MX, et al. Androgen receptor signaling regulates DNA repair in prostate cancers. Cancer Discov [Internet] 2013;3(11):1245–53. Disponible en: https://doi.org/10.1158/2159-8290.Cd-13-0172
- Pilié PG, Gay CM, Byers LA, et al. PARP Inhibitors: Extending Benefit Beyond BRCA-Mutant Cancers. Clin Cancer Res [Internet] 2019;25(13):3759–71.Disponible en: https://doi.org/10.1158/1078-0432.Ccr-18-0968
- Saad F, Clarke NW, Oya M, et al. Olaparib plus abiraterone versus placebo plus abiraterone in metastatic castration-resistant prostate cancer (PROpel): final prespecified overall survival results of a randomised, double-blind, phase 3 trial. Lancet Oncol [Internet] 2023;24(10):1094–108.Disponible en: https://doi.org/10.1016/s1470-2045(23)00382-0
- Agarwal N, Azad A, Carles J, et al. Final overall survival (OS) with talazoparib (TALA) + enzalutamide (ENZA) as first-line treatment in unselected patients with metastatic castration-resistant prostate cancer (mCRPC) in the phase 3 TALAPRO-2 trial. Journal of Clinical Oncology [Internet] 2025;43(5_suppl):LBA18–LBA18.Disponible en: https://doi.org/10.1200/JCO.2025.43.5_suppl.LBA18
- Agarwal N, Saad F, Azad A, et al. TALAPRO-3: A phase 3, double-blind, randomized study of enzalutamide (ENZA) plus talazoparib (TALA) versus placebo plus ENZA in patients with DDR gene–mutated, metastatic castration-sensitive prostate cancer (mCSPC). Journal of Clinical Oncology [Internet] 2022;40(16_suppl):TPS5096–TPS96.Disponible en: https://doi.org/10.1200/JCO.2022.40.16_suppl.TPS5096
- Rathkopf DE, Chi KN, Olmos D, et al. AMPLITUDE: A study of niraparib in combination with abiraterone acetate plus prednisone (AAP) versus AAP for the treatment of patients with deleterious germline or somatic homologous recombination repair (HRR) gene-altered metastatic castration-sensitive prostate cancer (mCSPC). Journal of Clinical Oncology [Internet] 2021;39(6_suppl):TPS176–TPS76.Disponible en: https://doi.org/10.1200/JCO.2021.39.6_suppl.TPS176
- Herencia-Ropero A, Llop-Guevara A, Staniszewska AD, et al. The PARP1 selective inhibitor saruparib (AZD5305) elicits potent and durable antitumor activity in patient-derived BRCA1/2-associated cancer models. Genome Med [Internet] 2024;16(1):107.Disponible en: https://doi.org/10.1186/s13073-024-01370-z
- Yap TA, Im S-A, Schram AM, et al. Abstract CT007: PETRA: First in class, first in human trial of the next generation PARP1-selective inhibitor AZD5305 in patients (pts) with BRCA1/2, PALB2 or RAD51C/D mutations. Cancer research [Internet] 2022;82(12_Supplement):CT007–CT07. Disponible en: https://doi.org/10.1158/1538-7445.Am2022-ct007
- Chi KN, Agarwal N, Armstrong AJ, et al. Phase III, double-blind, placebo-controlled, 2-cohort, randomized study of saruparib (AZD5305) in combination with new hormonal agents in patients with metastatic castration-sensitive prostate cancer with and without homologous recombination repair mutation (EvoPAR-Prostate01). Journal of Clinical Oncology [Internet] 2024;42(16_suppl):TPS5123–TPS23. Disponible en: https://doi.org/10.1200/JCO.2024.42.16_suppl.TPS5123
- Mei L, Zhang J, He K, et al. Ataxia telangiectasia and Rad3-related inhibitors and cancer therapy: where we stand. J Hematol Oncol [Internet] 2019;12(1):43. Disponible en: https://doi.org/10.1186/s13045-019-0733-6
- Reichert ZR, Devitt ME, Alumkal JJ, et al. Targeting resistant prostate cancer, with or without DNA repair defects, using the combination of ceralasertib (ATR inhibitor) and olaparib (the TRAP trial). Journal of Clinical Oncology [Internet] 2022;40(6_suppl):88–88. Disponible en: https://doi.org/10.1200/JCO.2022.40.6_suppl.088
- Tang Z, Pilié PG, Geng C, et al. ATR Inhibition Induces CDK1-SPOP Signaling and Enhances Anti-PD-L1 Cytotoxicity in Prostate Cancer. Clin Cancer Res [Internet] 2021;27(17):4898–909.Disponible en: https://doi.org/10.1158/1078-0432.Ccr-21-1010
- Choudhury AD. PTEN-PI3K pathway alterations in advanced prostate cancer and clinical implications. Prostate [Internet] 2022;82 Suppl 1:S60–s72. Disponible en: https://doi.org/10.1002/pros.24372
- Sweeney C, Bracarda S, Sternberg CN, et al. Ipatasertib plus abiraterone and prednisolone in metastatic castration-resistant prostate cancer (IPATential150): a multicentre, randomised, double-blind, phase 3 trial. Lancet [Internet] 2021;398(10295):131–42. Disponible en: https://doi.org/10.1016/s0140-6736(21)00580-8
- Shore N, Mellado B, Shah S, et al. A Phase I Study of Capivasertib in Combination With Abiraterone Acetate in Patients With Metastatic Castration-Resistant Prostate Cancer. Clin Genitourin Cancer [Internet] 2023;21(2):278–85. Disponible en: https://doi.org/10.1016/j.clgc.2022.11.017
- Bakht MK, Yamada Y, Ku SY, et al. Landscape of prostate-specific membrane antigen heterogeneity and regulation in AR-positive and AR-negative metastatic prostate cancer. Nat Cancer [Internet] 2023;4(5):699–715. Disponible en: https://doi.org/10.1038/s43018-023-00539-6
- Sood A, Kishan UA, Evans PC, et al. The Impact of Positron Emission Tomography Imaging and Tumor Molecular Profiling on Risk Stratification, Treatment Choice, and Oncological Outcomes of Patients with Primary or Relapsed Prostate Cancer: An International Collaborative Review of the Existing. European Urology Oncology [Internet] 2024;7(1):27–43. Disponible en: https://doi.org/10.1016/j.euo.2023.06.002
- Sartor O, Bono DJ, Chi NK, et al. Lutetium-177–PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. New England Journal of Medicine [Internet] 2021;385(12):1091–103. Disponible en: https://doi.org/10.1056/NEJMoa2107322
- Morris JM, Castellano D, Herrmann K, et al. 177Lu-PSMA-617 versus a change of androgen receptor pathway inhibitor therapy for taxane-naive patients with progressive metastatic castration-resistant prostate cancer (PSMAfore): a phase 3, randomised, controlled trial. The Lancet [Internet] 2024;404(10459):1227–39. Disponible en: https://doi.org/10.1016/S0140-6736(24)01653-2
- Ayzman A, Pachynski KR, Reimers AM. PSMA-based Therapies and Novel Therapies in Advanced Prostate Cancer: The Now and the Future. Current Treatment Options in Oncology [Internet] 2025;26(5):375–84. Disponible en: https://doi.org/10.1007/s11864-025-01317-5
- Zarrabi KK, Narayan V, Mille PJ, et al. Bispecific PSMA antibodies and CAR-T in metastatic castration-resistant prostate cancer. Therapeutic Advances in Urology [Internet] 2023;15. Disponible en: https://doi.org/10.1177/17562872231182219
- Bhatia V, Kamat NV, Pariva TE, et al. Targeting advanced prostate cancer with STEAP1 chimeric antigen receptor T cell and tumor-localized IL-12 immunotherapy. Nat Commun [Internet] 2023;14(1):2041. Disponible en: https://doi.org/10.1038/s41467-023-37874-2
- Mizuno K, Beltran H. Future directions for precision oncology in prostate cancer. Prostate [Internet] 2022;82 Suppl 1(Suppl 1):S86–s96. Disponible en: https://doi.org/10.1002/pros.24354
- Urabe F, Sumiyoshi T, Tashiro K, et al. Prostate cancer and liquid biopsies: Clinical applications and challenges. Int J Urol [Internet] 2024;31(6):617–26. Disponible en: https://doi.org/10.1111/iju.15441

















