Políticas públicas para la integración de la biología molecular tumoral en la práctica oncológica: lecciones de los marcos internacionales y una hoja de ruta para América Latina
Public policy for integrating molecular tumor biology in oncology practice: lessons from international frameworks and a roadmap for Latin America
Cómo citar
Descargar cita

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-SinDerivadas 4.0.
Mostrar biografía de los autores
Antecedentes: los avances en biología molecular tumoral han transformado la oncología al permitir enfoques de intervención basados en biomarcadores para el diagnóstico, el pronóstico y el tratamiento. Sin embargo, la integración de estas innovaciones en la práctica clínica habitual depende en gran medida de los marcos de políticas públicas que regulan la validación de pruebas, el reembolso y el acceso equitativo.
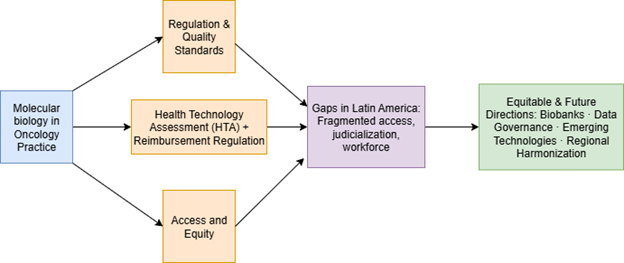
Objetivo: esta revisión examina las experiencias internacionales sobre políticas relativas al uso de biomarcadores en oncología, evalúa el panorama actual en América Latina y propone una hoja de ruta para la adopción equitativa de la biología molecular tumoral en la región.
Enfoque: se realizó una revisión narrativa de la literatura revisada por pares y de documentos de políticas de referencia. Se analizaron los marcos regulatorios de Estados Unidos, Canadá y la Unión Europea para estrategias de reembolso y evaluación de tecnologías sanitarias (ETS). La evidencia latinoamericana se sintetizó a partir de estudios publicados e informes regionales sobre cobertura, judicialización e infraestructura.
Resultados: en los países de altos ingresos, la integración de biomarcadores se apoya en una sólida supervisión regulatoria, una ETS estructurada y mecanismos de reembolso, aunque el acceso sigue siendo desigual. En América Latina, el acceso está fragmentado, con importantes disparidades entre los sectores público y privado. Las barreras incluyen reembolsos limitados, infraestructura de laboratorios deficiente, escasez de personal y la ausencia de marcos regulatorios estandarizados. La judicialización se ha convertido en una vía de acceso común, pero inequitativa. Existen oportunidades para aprovechar las colaboraciones regionales, el desarrollo de biobancos y la gobernanza armonizada de datos.
Conclusiones: la innovación en políticas es esencial para garantizar una oncología de precisión equitativa en América Latina. Se propone una hoja de ruta en etapas que abarca la claridad regulatoria, el reembolso piloto, la capacitación del personal y la armonización regional a largo plazo. La preparación para tecnologías emergentes, como la biopsia líquida y las terapias agnósticas para tumores, será fundamental para evitar el aumento de las disparidades y aprovechar al máximo el potencial de la biología molecular tumoral en la región.
Visitas del artículo 0 | Visitas PDF 0
Descargas
- Mathur S, Sutton J. Personalized medicine could transform healthcare. Biomed Rep [Internet]. 2017;7(1):3–5. Available from: https://doi.org/10.3892/br.2017.922
- Zhou Y, Tao L, Qiu J, Xu J, Yang X, Zhang Y, et al. Tumor biomarkers for diagnosis, prognosis and targeted therapy. Signal Transduct Target Ther [Internet]. 2024;9(1):132. Available from: https://www.nature.com/articles/s41392-024-01823-2
- Schilsky RL. Personalized medicine in oncology: the future is now. Nat Rev Drug Discov [Internet]. 2010;9(5):363–6. Available from: https://www.nature.com/articles/nrd3181
- Purkayastha K, Dhar R, Pethusamy K, Srivastava T, Shankar A, Rath GK, et al. The issues and challenges with cancer biomarkers. J Cancer Res Ther [Internet]. 2023;19(Supplement):S20–35. Available from: https://doi.org/10.4103/jcrt.jcrt_384_22
- Zou J, Wang E. Cancer Biomarker Discovery for Precision Medicine: New Progress. Curr Med Chem [Internet]. 2019;26(42):7655–71. Available from: https://doi.org/10.2174/0929867325666180718164712
- Centers for Medicare & Medicaid Services. Next Generation Sequencing (NGS) for Medicare Beneficiaries with Advanced Cancer [Internet]. 2018. Report No.: CAG-00450N. Available from: https://www.cms.gov/medicare-coverage-database/view/ncacal-decision-memo.aspx?proposed=N&ncaid=296&ExpandComments=n&NCDId=342&ncdver=1&SearchType=Advanced&CoverageSelection=National&NCSelection=NCA%7CNCD%7CMCD&KeyWord=uspstf&KeyWordLookUp=Doc&KeyWordSearchType=Exact&kq=true&bc=IAAAADgAAAAA
- Normanno N, Apostolidis K, Wolf A, Al Dieri R, Deans Z, Fairley J, et al. Access and quality of biomarker testing for precision oncology in Europe. Eur J Cancer Oxf Engl [Internet]. 2022;176:70–7. Available from: https://doi.org/10.1016/j.ejca.2022.09.005
- Westphalen CB, Boscolo Bielo L, Aftimos P, Beltran H, Benary M, Chakravarty D, et al. ESMO Precision Oncology Working Group recommendations on the structure and quality indicators for molecular tumour boards in clinical practice. Ann Oncol Off J Eur Soc Med Oncol [Internet]. 2025;36(6):614–25. Available from: https://doi.org/10.1016/j.annonc.2025.02.009
- European Commission. Regulation on on health technology assessment [Internet]. 2021. Report No.: 2021/2282. Available from: https://eur-lex.europa.eu/eli/reg/2021/2282/oj/eng
- CADTH Advisory Panel. Planning for a Coordinated Assessment Framework for Biomarkers Used in Cancer Care: A Report from the Biomarker Advisory Panel [Internet]. 2025. Report No.: CP0048-000. Available from: https://www.cda-amc.ca/sites/default/files/2025-08/CP0048-Biomarker-Assessment-Framework_DRAFT_V1.0_EN.pdf
- Govaerts L, Simoens S, Van Dyck W, Huys I. Shedding Light on Reimbursement Policies of Companion Diagnostics in European Countries. Value Health J Int Soc Pharmacoeconomics Outcomes Res. 2020;23(5):606–15. Available from: https://doi.org/10.1016/j.jval.2020.01.013
- DaCosta Byfield S, Bapat B, Becker L, Reyes C, Chatzitheofilou I, Schroeder BE, et al. Biomarker Testing Approaches, Treatment Selection, and Cost of Care Among Adults With Advanced Cancer. JAMA Netw Open [Internet]. 2025;8(7):e2519963. Available from: https://doi.org/10.1001/jamanetworkopen.2025.19963
- Mirza M, Goerke L, Anderson A, Wilsdon T. Assessing the Cost-Effectiveness of Next-Generation Sequencing as a Biomarker Testing Approach in Oncology and Policy Implications: A Literature Review. Value Health [Internet]. 2024;27(9):1300–9. Available from: https://doi.org/10.1016/j.jval.2024.04.023
- Piñeros M, Laversanne M, Barrios E, Cancela M de C, de Vries E, Pardo C, et al. An updated profile of the cancer burden, patterns and trends in Latin America and the Caribbean. Lancet Reg Health Am [Internet]. 2022;13. Available from: https://doi.org/10.1016/j.lana.2022.100294
- Schneider LP, Maselli-Schoueri JH, Gutierres Aguiar B de S, Nazareth Aguiar P, Del Giglio A. Addressing challenges in the implementation of precision oncology: An in-depth examination of limitations and disparities in the treatment of non-small cell lung cancer in the Brazilian public healthcare system (SUS). Glob Public Health [Internet]. 2025;20(1):2450412. Available from: https://doi.org/10.1080/17441692.2025.2450412
- López Rivera JJ, Rueda-Gaitán P, Rios Pinto LC, Rodríguez Gutiérrez DA, Gomez-Lopera N, Lamilla J, et al. Advancing Cancer Care in Colombia: Results of the First In Situ Implementation of Comprehensive Genomic Profiling. J Pers Med [Internet]. 2024;14(9):975. Available from: https://doi.org/10.3390/jpm14090975
- Vargas-Pelaez CM, Rover MRM, Soares L, Blatt CR, Mantel-Teeuwisse AK, Rossi FA, et al. Judicialization of access to medicines in four Latin American countries: a comparative qualitative analysis. Int J Equity Health [Internet]. 2019;18(1):68. Available from: https://doi.org/10.1186/s12939-019-0960-z
- González AR, Merchán LAA, Alexander JAA, Kaen D, Lopez-Correa C, Martin C, et al. Precision Medicine for Cancer and Health Equity in Latin America: Generating Understanding for Policy and Health System Shaping. Int J Environ Res Public Health [Internet]. 2025;22(8):1220. Available from: https://www.mdpi.com/1660-4601/22/8/1220
- de Castilla EMR, Mayrides M, González H, Vidangossy F, Corbeaux T, Ortiz N, et al. Implementing precision oncology in Latin America to improve patient outcomes: the status quo and a call to action for key stakeholders and decision-makers. Ecancermedicalscience [Internet]. 2024;18:1653. Available from: https://doi.org/10.3332/ecancer.2024.1653
- Llera AS. A fresh perspective on Latin America cancer care: uncovering hidden messages in unconventional data sources. Lancet Reg Health Am [Internet]. 2023;24:100559. Available from: https://doi.org/10.1016/j.lana.2023.100559
- Torres-Narvaez ES, Mendivelso-González DF, Artunduaga-Alvarado JA, Ortega-Recalde O. Cancer genomics and bioinformatics in Latin American countries: applications, challenges, and perspectives. Front Oncol [Internet]. 2025;15:1584178. Available from: https://doi.org/10.3389/fonc.2025.1584178
- CMS. Next Generation Sequencing (NGS): Testing in advanced cancers [Internet]. 2018. Report No.: 100–3. Available from: https://www.cms.gov/Regulations-and-Guidance/Guidance/Transmittals/2019Downloads/R215NCD.pdf
- Sheinson DM, Wong WB, Flores C, Ogale S, Gross CP. Association Between Medicare’s National Coverage Determination and Utilization of Next-Generation Sequencing. JCO Oncol Pract [Internet]. 2021;17(11):e1774–84. Available from: https://ascopubs.org/doi/10.1200/OP.20.01023
- Trosman JR, Weldon CB, Gradishar WJ, Benson AB, Cristofanilli M, Kurian AW, et al. From the Past to the Present: Insurer Coverage Frameworks for Next-Generation Tumor Sequencing. Value Health J Int Soc Pharmacoeconomics Outcomes Res [Internet]. 2018;21(9):1062–8. Available from: https://doi.org/10.1016/j.jval.2018.06.011
- Trosman JR, Weldon CB, Douglas MP, Kurian AW, Kelley RK, Deverka PA, et al. Payer Coverage for Hereditary Cancer Panels: Barriers, Opportunities, and Implications for the Precision Medicine Initiative. J Natl Compr Cancer Netw JNCCN [Internet]. 2017;15(2):219–28. Available from: https://doi.org/10.6004/jnccn.2017.0022
- CMS. MolDX: Phenotypic Biomarker Detection in Circulating Tumor Cells. Report No.: LCD ID L38566. 2021. Available from: https://www.cms.gov/medicare-coverage-database/view/lcd.aspx?lcdid=38566
- O’Dwyer PJ, Gray RJ, Flaherty KT, Chen AP, Li S, Wang V, et al. The NCI-MATCH trial: lessons for precision oncology. Nat Med [Internet]. 2023;29(6):1349–57. Available from: https://doi.org/10.1038/s41591-023-02379-4
- Kaye J, Briceño Moraia L, Mitchell C, Bell J, Bovenberg JA, Tassé AM, et al. Access Governance for Biobanks: The Case of the BioSHaRE-EU Cohorts. Biopreservation Biobanking [Internet]. 2016 June;14(3):201–6. Available from: https://doi.org/10.1089/bio.2015.0124
- Reihs R, Proynova R, Maqsood S, Ataian M, Lablans M, Quinlan PR, et al. BBMRI-ERIC Negotiator: Implementing Efficient Access to Biobanks. Biopreservation Biobanking [Internet]. 2021;19(5):414–21. Available from: https://doi.org/10.1089/bio.2020.0144
- Knoppers BM. Framework for responsible sharing of genomic and health-related data. HUGO J [Internet]. 2014;8(1):3. Available from: https://doi.org/10.1186/s11568-014-0003-1
- Dienstmann R. WS04.02 Access to Biomarker Testing in Latin America. J Thorac Oncol [Internet]. 2021;16(10):S842. Available from: https://doi.org/10.1016/j.jtho.2021.08.759
- Arai RJ, Guindalini RSC, Llera AS, O’Connor JM, Muller B, Lema M, et al. Personalizing Precision Oncology Clinical Trials in Latin America: An Expert Panel on Challenges and Opportunities. The Oncologist [Internet]. 2019;24(8):e709–19. Available from: https://doi.org/ 10.1634/theoncologist.2018-0318
- Ginsburg GS, Phillips KA. Precision Medicine: From Science To Value. Health Aff Proj Hope [Internet]. 2018;37(5):694–701. Available from: https://doi.org/10.1377/hlthaff.2017.1624
- Alvarado-Cabrero I, Doimi F, Ortega V, de Oliveira Lima JT, Torres R, Torregrosa L. Recommendations for streamlining precision medicine in breast cancer care in Latin America. Cancer Rep Hoboken NJ [Internet]. 2021;4(6):e1400. Available from: https://doi.org/10.1002/cnr2.1400
- International Agency for Research on Cancer Global Cancer Observatory in Brazil. 2024. Available from: https://gco.iarc.who.int/media/globocan/factsheets/populations/76-brazil-fact-sheet.pdf.
- Duarte FA, Ferreira CG, Dienstmann R, Ferrari BL, Costa E Silva M, Nazareth A Junior P, et al. Barriers in precision medicine implementation among Advanced Nonsquamous Cell Lung Cancer-patients: A Real-World Evidence Scenario. J Mark Access Health Policy [Internet]. 2022;10(1):2077905. Available from: https://doi.org/10.1080/20016689.2022.2077905
- World Health Organization. Health technology assessment of medical devices [Internet]. 2024. Report No.: 2. Available from: https://iris.who.int/server/api/core/bitstreams/68a45b5a-97a6-44e1-b3bf-8cb39cc460f3/content
- Eichler HG, Trusheim M, Schwarzer-Daum B, Larholt K, Zeitlinger M, Brunninger M, et al. Precision Reimbursement for Precision Medicine: Using Real-World Evidence to Evolve From Trial-and-Project to Track-and-Pay to Learn-and-Predict. Clin Pharmacol Ther [Internet]. 2022;111(1):52–62. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1002/cpt.2471
- Moye-Holz D, Soria Saucedo R, van Dijk JP, Reijneveld SA, Hogerzeil HV. Access to innovative cancer medicines in a middle-income country - the case of Mexico. J Pharm Policy Pract [Internet]. 2018;11:25. Available from: https://doi.org/10.1186/s40545-018-0153-y
- Mapa de accionabilidad genómica tumoral argentina (MAGenTa) [Internet]. Argentina.gob.ar. 2020. Available from: https://www.argentina.gob.ar/ciencia/dnpe/proyectos/medicina-precision/MAGenTa
- Fernández-Ramires R, Morales-Pison S, Rucatti GG, Echeverría C, San Martín E, Cammarata-Scalisi F, et al. Cancer genetic counseling in Chile: Addressing barriers, confronting challenges, and seizing opportunities in an underserved Latin American Community. Genet Med Open [Internet]. 2024;2(Suppl 2):101898. Available from: https://doi.org/10.1016/j.gimo.2024.101898
- PAHO/WHO, Pan American Health Organization. Latin America and the Caribbean Code against Cancer [Internet]. 2023. Available from: https://www.paho.org/en/latin-america-and-caribbean-code-against-cancer
- Argudo-Portal V, Domènech M. The reconfiguration of biobanks in Europe under the BBMRI-ERIC framework: towards global sharing nodes? Life Sci Soc Policy [Internet]. 2020 Oct 1 [cited 2025 Sept 19];16(1):9. Available from: https://doi.org/10.1186/s40504-020-00105-3
- International Society for Biological and Environmental Repositories (ISBER). Best Practices for Repositories [Internet]. 2025. Report No.: Fifth Edition. Available from: https://www.isber.org/page/BPR
- Otten LS, Buma AIG, Piet B, ter Heine R, van den Heuvel MM, Retèl VP. Very Early Health Technology Assessment for Potential Predictive Biomarkers in the Treatment of Advanced Non-Small Cell Lung Cancer. PharmacoEconomics - Open [Internet]. 2025;9(3):471–85. Available from: https://doi.org/10.1007/s41669-025-00557-3
- Ferraro S, Biganzoli EM, Castaldi S, Plebani M. Health Technology Assessment to assess value of biomarkers in the decision-making process. Clin Chem Lab Med [Internet]. 2022;60(5):647–54. Available from: https://doi.org/ 10.1515/cclm-2021-1291
- Herrero Colomina J, Johnston E, Duffus K, Zaïr ZM, Thistlethwaite F, Krebs M, et al. Real-world experience of Molecular Tumour Boards for clinical decision-making for cancer patients. NPJ Precis Oncol [Internet]. 2025;9:87. Available from: https://doi.org/10.1038/s41698-025-00863-3
- Anaya JM, Herrán M, Pino LE. Challenges and opportunities for precision medicine in developing nations. Expert Rev Precis Med Drug Dev [Internet]. 2025;10(1):1–15. Available from: https://doi.org/10.1080/23808993.2025.2505796
- Horgan D, Ciliberto G, Conte P, Curigliano G, Seijo L, Montuenga LM, et al. Bringing Onco-Innovation to Europe’s Healthcare Systems: The Potential of Biomarker Testing, Real World Evidence, Tumour Agnostic Therapies to Empower Personalised Medicine. Cancers [Internet]. 2021;13(3):583. Available from: https://doi.org/10.3390/cancers13030583

















