Biología molecular del cáncer de pulmón de célula no pequeña
Molecular biology of Non-small cell lung cancer
Cómo citar
Descargar cita

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-SinDerivadas 4.0.
Mostrar biografía de los autores
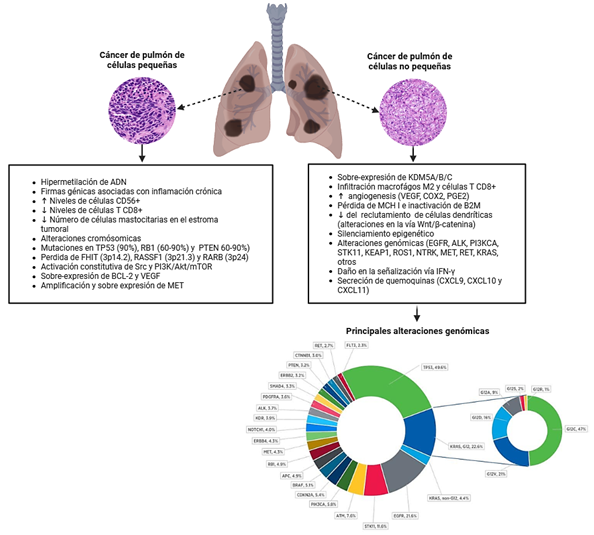
Introducción: el cáncer de pulmón de célula no pequeña (NSCLC) representa aproximadamente el 85% de los casos de cáncer de pulmón y sigue siendo una causa principal de mortalidad por cáncer a nivel mundial, con una tasa de supervivencia global a 5 años de alrededor del 20%.
Métodos: se realizó una revisión narrativa de la literatura que sintetiza el conocimiento actual sobre la biología molecular del NSCLC, enfatizando las alteraciones genómicas, la evolución tumoral y las implicaciones terapéuticas, particularmente en el adenocarcinoma (LUAD) y el carcinoma de células escamosas (LUSC).
Resultados: se destacan diferencias clave entre fumadores y no fumadores, incluyendo una mayor carga mutacional y transversiones en fumadores, frente a alteraciones genómicas accionables (AGAs) como EGFR, KRAS, ALK y ROS1 en no fumadores. La revisión discute cambios somáticos en el ADN, perfiles de expresión de ARN, comutaciones, microambiente tumoral (TME), vesículas extracelulares y enfoques multiómicos que revelan la heterogeneidad tumoral y los mecanismos de resistencia. Los aspectos traslacionales cubren diagnósticos emergentes, como el ADN tumoral circulante y los exosomas, así como terapias, incluyendo inhibidores de tirosina quinasa (TKIs), inmunoterapia y estrategias novedosas dirigidas al microbioma y a las microvesículas.
Conclusiones: la caracterización molecular del NSCLC resulta de la máxima relevancia para la toma de decisiones terapéuticas más allá de la enfermedad avanzada; a pesar de los avances, persisten brechas, subrayando la necesidad de enfoques personalizados para lograr la curación en etapas tempranas y mejorar los resultados en enfermedad avanzada.
Visitas del artículo 0 | Visitas PDF 0
Descargas
- Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin [Internet]. 2024;74(3):229-263. Disponible en: https://doi.org/10.3322/caac.21834
- Cardona AF, Mejía SA, Viola L, Chamorro DF, Rojas L, Ruíz-Patiño A, et al. Lung Cancer in Colombia. J Thorac Oncol [Internet]. 2022;17(8):953-960. Disponible en: https://doi.org/10.1016/j.jtho.2022.02.015
- Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin [Internet]. 2024;74(3):229-263. Disponible en: https://doi.org/10.3322/caac.21834
- Jani CT, Kareff SA, Morgenstern-Kaplan D, Salazar AS, Hanbury G, Salciccioli JD, et al. Evolving trends in lung cancer risk factors in the ten most populous countries: an analysis of data from the 2019 Global Burden of Disease Study. EClinicalMedicine [Internet]. 2025;79:103033. Disponible en: https://doi.org/10.1016/j.eclinm.2024.103033
- Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: Epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc [Internet]. 2008;83(5):584-594. Disponible en: https://doi.org/10.4065/83.5.584
- Yano T, Miura N, Takenaka T, Haro A, Okazaki H, Ohba T, et al. Never-smoking nonsmall cell lung cancer as a separate entity: Clinicopathologic features and survival. Cancer [Internet]. 2008;113(5):1012-1018. Disponible en: https://doi.org/10.1002/cncr.23679
- Toh CK, Gao F, Lim WT, Leong SS, Fong KW, Yap SP, et al. Never-smokers with lung cancer: Epidemiologic evidence of a distinct disease entity. J Clin Oncol [Internet]. 2006;24(15):2245-2251. Disponible en: https://doi.org/10.1200/JCO.2005.04.8033
- Barlesi F, Mazieres J, Merlio JP, Debieuvre D, Mosser J, Lena H, et al. Routine molecular profiling of patients with advanced non-small-cell lung cancer: Results of a 1-year nationwide programme of the French Cooperative Thoracic Intergroup (IFCT). Lancet [Internet]. 2016;387(10026):1415-1426. Disponible en: https://doi.org/10.1016/S0140-6736(16)00004-0
- Chen H, Carrot-Zhang J, Zhao Y, Hu H, Freeman SS, Yu S, et al. Genomic and immune profiling of pre-invasive lung adenocarcinoma. Nat Commun [Internet]. 2019;10(1):5472. Disponible en: https://doi.org/10.1038/s41467-019-13460-3
- Lengel HB, Mastrogiacomo B, Connolly JG, Tan KS, Liu Y, Fick CN, et al. Genomic mapping of metastatic organotropism in lung adenocarcinoma. Cancer Cell [Internet]. 2023;41(5):970-985.e7. Disponible en: https://doi.org/10.1016/j.ccell.2023.03.018
- Gerstberger S, Jiang Q, Ganesh K. Metastasis. Cell [Internet]. 2023;186(8):1564-1579. Disponible en: https://doi.org/10.1016/j.cell.2023.03.003
- Collisson EA, Campbell JD, Brooks AN, Berger AH, Lee W, Chmielecki J, et al. Comprehensive molecular profiling of lung adenocarcinoma: The Cancer Genome Atlas Research Network. Nature [Internet]. 2014;511(7511):543-550. Disponible en: https://doi.org/10.1038/nature13385
- Hammerman PS, Voet D, Lawrence MS, Voet D, Jing R, Cibulskis K, et al. Comprehensive genomic characterization of squamous cell lung cancers. Nature [Internet]. 2012;489(7417):519-525. Disponible en: https://doi.org/10.1038/nature11404
- Naranbhai V, Viard M, Dean M, Groha S, Braun DA, Labaki C, et al. HLA-A*03 and response to immune checkpoint blockade in cancer: an epidemiological biomarker study. Lancet Oncol [Internet]. 2022;23(1):172-184. Disponible en: https://doi.org/10.1016/S1470-2045(21)00582-9
- Ahrendt SA, Hu Y, Buta M, McDermott MP, Benoit N, Yang SC, et al. p53 mutations and survival in stage I non-small-cell lung cancer: Results of a prospective study. J Natl Cancer Inst [Internet]. 2003;95(13):961-970. Disponible en: https://doi.org/10.1093/jnci/95.13.961
- Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature [Internet]. 2018;553(7689):446-454. Disponible en: https://doi.org/10.1038/nature25183
- Esfahani MS, Lee LJ, Jeon YJ, Flynn RA, Stehr H, Hui AB, et al. Functional significance of U2AF1 S34F mutations in lung adenocarcinomas. Nat Commun [Internet]. 2019;10(1):5712. Disponible en: https://doi.org/10.1038/s41467-019-13392-y
- Hill W, Lim EL, Weeden CE, Lee C, Augustine M, Chen K, et al. Lung adenocarcinoma promotion by air pollutants. Nature [Internet]. 2023;616(7955):159-167. Disponible en: https://doi.org/10.1038/s41586-023-05874-3
- Arrieta O, Cardona AF, Martín C, Más-López L, Corrales-Rodríguez L, Bramuglia G, et al. Updated frequency of EGFR and KRAS mutations in NonSmall-cell lung cancer in Latin America: The Latin-American consortium for the investigation of lung cancer (CLICaP). J Thorac Oncol [Internet]. 2015;10(5):838-843. Disponible en: https://doi.org/10.1097/JTO.0000000000000481
- Saldanha EF, Cordeiro de Lima VC, Fares A, Corassa M, Gil-Santana L, Arrieta O, et al. Real-world characteristics and outcomes of ERBB2-mutant NSCLC in Latin American patients (CLICaP). Oncologist [Internet]. 2025;30(2). Disponible en: https://doi.org/10.1093/oncolo/oyae347
- Fois SS, Paliogiannis P, Zinellu A, Fois AG, Cossu A, Palmieri G. Molecular epidemiology of the main druggable genetic alterations in non-small cell lung cancer. Int J Mol Sci [Internet]. 2021;22(2):612. Disponible en: https://doi.org/10.3390/ijms22020612
- Voena C, Ambrogio C, Iannelli F, Chiarle R. ALK in cancer: from function to therapeutic targeting. Nat Rev Cancer [Internet]. 2025;25(5):359-378. Disponible en: https://doi.org/10.1038/s41568-025-00797-9
- Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature [Internet]. 2018;553(7689):446-454. Disponible en: https://doi.org/10.1038/nature25183
- Zha Z, Liu C, Yan M, Chen C, Yu C, Chen Y, et al. RNase1-driven ALK-activation is an oncogenic driver and therapeutic target in non-small cell lung cancer. Signal Transduct Target Ther [Internet]. 2025;10(1):124. Disponible en: https://doi.org/10.1038/s41392-025-02206-x
- Reva B, Antipin Y, Sander C. Predicting the functional impact of protein mutations: Application to cancer genomics. Nucleic Acids Res [Internet]. 2011;39(17):e118. Disponible en: https://doi.org/10.1093/nar/gkr407
- Chen M, Xu Y, Zhao J, Zhong W, Zhang L, Bi Y, et al. Concurrent Driver Gene Mutations as Negative Predictive Factors in Epidermal Growth Factor Receptor-Positive Non-Small Cell Lung Cancer. EBioMedicine [Internet]. 2019;42:304-310. Disponible en: https://doi.org/10.1016/j.ebiom.2019.03.023
- Shigematsu H, Lin L, Takahashi T, Nomura M, Suzuki M, Wistuba II, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst [Internet]. 2005;97(5):339-346. Disponible en: https://doi.org/10.1093/jnci/dji055
- Chen M, Xu Y, Zhao J, Zhong W, Zhang L, Bi Y, et al. Concurrent Driver Gene Mutations as Negative Predictive Factors in Epidermal Growth Factor Receptor-Positive Non-Small Cell Lung Cancer. EBioMedicine [Internet]. 2019;42:304-310. Disponible en: https://doi.org/10.1016/j.ebiom.2019.03.023
- Galetta D, Catino A, Misino A. Concomitant EGFR mutations/ALK rearrangements: Beyond a simple dual target. Transl Lung Cancer Res [Internet]. 2016;5(1):143-144. Disponible en: https://doi.org/10.3978/j.issn.2218-6751.2016.01.09
- Lo Russo G, Imbimbo M, Corrao G, Proto C, Signorelli D, Vitali M, et al. Concomitant EML4-ALK rearrangement and EGFR mutation in non-small cell lung cancer patients: A literature review of 100 cases. Oncotarget [Internet]. 2017;8(35):59889-59900. Disponible en: https://doi.org/10.18632/oncotarget.17431
- Baldi L, Mengoli MC, Bisagni A, Banzi MC, Boni C, Rossi G. Concomitant EGFR mutation and ALK rearrangement in lung adenocarcinoma is more frequent than expected: Report of a case and review of the literature with demonstration of genes alteration into the same tumor cells. Lung Cancer [Internet]. 2014;86(2):291-295. Disponible en: https://doi.org/10.1016/j.lungcan.2014.09.011
- Awad MM, Liu S, Rybkin II, Arbour KC, Dilly J, Zhu VW, et al. Acquired Resistance to KRAS G12C Inhibition in Cancer. N Engl J Med [Internet]. 2021;384(25):2382-2393. Disponible en: https://doi.org/10.1056/NEJMoa2105281
- Chmielecki J, Gray JE, Cheng Y, Ohe Y, Imamura F, Cho BC, et al. Candidate mechanisms of acquired resistance to first-line osimertinib in EGFR-mutated advanced non-small cell lung cancer. Nat Commun [Internet]. 2023;14(1):1070. Disponible en: https://doi.org/10.1038/s41467-023-35961-y
- Isermann T, Sers C, Der CJ, Papke B. KRAS inhibitors: resistance drivers and combinatorial strategies. Trends Cancer [Internet]. 2025;11(2):91-116. Disponible en: https://doi.org/10.1016/j.trecan.2024.11.009
- Scheffler M, Ihle MA, Hein R, Merkelbach-Bruse S, Scheel AH, Siemanowski J, et al. K-ras Mutation Subtypes in NSCLC and Associated Co-occuring Mutations in Other Oncogenic Pathways. J Thorac Oncol [Internet]. 2019;14(4):606-616. Disponible en: https://doi.org/10.1016/j.jtho.2018.12.013
- Tu X, Lu Z, Hei F, Zhang T, Wang X, Chen D, et al. Putative mechanisms of primary resistance to EGFR-targeted therapies: A retrospective study. Lung Cancer [Internet]. 2024;197:107942. Disponible en: https://doi.org/10.1016/j.lungcan.2024.107998
- Gandhi MM, Elkrief A, Moore CG, Ricciuti B, Alessi JV, Richards AL, et al. Gene Copy Deletion of STK11, KEAP1, and SMARCA4: Clinicopathologic Features and Association With the Outcomes of Immunotherapy With or Without Chemotherapy in Nonsquamous NSCLC. J Thorac Oncol [Internet]. 2025;20(6):725-738. Disponible en: https://doi.org/10.1016/j.jtho.2025.01.016
- Skoulidis F, Araujo HA, Do MT, Qian Y, Sun X, Cobo AG, et al. CTLA4 blockade abrogates KEAP1/STK11-related resistance to PD-(L)1 inhibitors. Nature [Internet]. 2024;635(8038):462-471. Disponible en: https://doi.org/10.1038/s41586-024-07943-7
- Bischoff P, Reck M, Overbeck T, Christopoulos P, Rittmeyer A, Lüders H, et al. Outcome of First-Line Treatment With Pembrolizumab According to KRAS/TP53 Mutational Status for Nonsquamous Programmed Death-Ligand 1–High (≥50%) NSCLC in the German National Network Genomic Medicine Lung Cancer. J Thorac Oncol [Internet]. 2024;19(5):803-817. Disponible en: https://doi.org/10.1016/j.jtho.2023.12.015
- Robinson DR, Wu YM, Lonigro RJ, Vats P, Cobain E, Everett J, et al. Integrative clinical genomics of metastatic cancer. Nature [Internet]. 2017;548(7667):297-303. Disponible en: https://doi.org/10.1038/nature23306
- McGranahan N, Furness AJS, Rosenthal R, Ramskov S, Lyngaa R, Saini SK, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science [Internet]. 2016;351(6280):1463-1469. Disponible en: https://doi.org/10.1126/science.aaf1490
- Koyama S, Akbay EA, Li YY, Aref AR, Skoulidis F, Herter-Sprie GS, et al. STK11/LKB1 deficiency promotes neutrophil recruitment and proinflammatory cytokine production to suppress T-cell activity in the lung tumor microenvironment. Cancer Res [Internet]. 2016;76(5):999-1008. Disponible en: https://doi.org/10.1158/0008-5472.CAN-15-1439
- Gillette MA, Satpathy S, Cao S, Dhanasekaran SM, Vasaikar SV, Krug K, et al. Proteogenomic Characterization Reveals Therapeutic Vulnerabilities in Lung Adenocarcinoma. Cell [Internet]. 2020;182(1):200-225.e35. Disponible en: https://doi.org/10.1016/j.cell.2020.06.013
- Lee LH, Xu X, Mourikis T, Tang F, Fairchild L, Ji L, et al. Defining Non–small Cell Lung Cancer Tumor Microenvironment Changes at Primary and Acquired Immune Checkpoint Inhibitor Resistance Using Clinical and Real-World Data. Cancer Res Commun [Internet]. 2025;5(6):1049-1059. Disponible en: https://doi.org/10.1158/2767-9764.crc-24-0605
- Zhao Y, Yang Z, Wu D, Zhao H. Dissecting the intratumoral microbiome landscape in lung cancer. Front Immunol [Internet]. 2025;16:1614731. Disponible en: https://doi.org/10.3389/fimmu.2025.1614731
- Doyle LM, Wang MZ. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells [Internet]. 2019;8(7):727. Disponible en: https://doi.org/10.3390/cells8070727
- Lin H, Zhou J, Ding T, Zhu Y, Wang L, Zhong T, et al. Therapeutic potential of extracellular vesicles from diverse sources in cancer treatment. Eur J Med Res [Internet]. 2024;29(1):350. Disponible en: https://doi.org/10.1186/s40001-024-01937-x
- Liu X, Jiang F, Wang Z, Tang L, Zou B, Xu P, et al. Hypoxic bone marrow mesenchymal cell-extracellular vesicles containing miR-328-3p promote lung cancer progression via the NF2-mediated Hippo axis. J Cell Mol Med [Internet]. 2021;25(1):96-109. Disponible en: https://doi.org/10.1111/jcmm.15865
- Santos P, Almeida F. Role of exosomal mirnas and the tumor microenvironment in drug resistance. Cells [Internet]. 2020;9(6):1450. Disponible en: https://doi.org/10.3390/cells9061450
- Zabeti Touchaei A, Norollahi SE, Najafizadeh A, Babaei K, Bakhshalipour E, Vahidi S, et al. Therapeutic combinations of exosomes alongside cancer stem cells (CSCs) and of CSC-derived exosomes (CSCEXs) in cancer therapy. Cancer Cell Int [Internet]. 2024;24(1):334. Disponible en: https://doi.org/10.1186/s12935-024-03514-y
- Deng Y, Zhang L, Luo R. LINC01783 facilitates cell proliferation, migration and invasion in non-small cell lung cancer by targeting miR-432-5p to activate the notch pathway. Cancer Cell Int [Internet]. 2021;21(1):234. Disponible en: https://doi.org/10.1186/s12935-021-01912-0
- Hosseini R, Asef-Kabiri L, Yousefi H, Sarvnaz H, Salehi M, Akbari ME, et al. The roles of tumor-derived exosomes in altered differentiation, maturation and function of dendritic cells. Mol Cancer [Internet]. 2021;20(1):83. Disponible en: https://doi.org/10.1186/s12943-021-01376-w
- Wang L, He J, Hu H, Tu L, Sun Z, Liu Y, et al. Lung CSC-derived exosomal miR-210-3p contributes to a pro-metastatic phenotype in lung cancer by targeting FGFRL1. J Cell Mol Med [Internet]. 2020;24(11):6324-6339. Disponible en: https://doi.org/10.1111/jcmm.15274
- Zhang Y, Fu F, Zhang Q, Li L, Liu H, Deng C, et al. Evolutionary proteogenomic landscape from pre-invasive to invasive lung adenocarcinoma. Cell Rep Med [Internet]. 2024;5(1):101358. Disponible en: https://doi.org/10.1016/j.xcrm.2023.101358
- Fukuoka M, Yano S, Giaccone G, Tamura T, Nakagawa K, Douillard JY, et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer. J Clin Oncol [Internet]. 2003;21(12):2237-2246. Disponible en: https://doi.org/10.1200/JCO.2003.10.038
- Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WEE, Poddubskaya E, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non–Small-Cell Lung Cancer. N Engl J Med [Internet]. 2015;373(2):123-135. Disponible en: https://doi.org/10.1056/NEJMoa1504627
- Blakely CM, Watkins TBK, Wu W, Gini B, Chabon JJ, McCoach CE, et al. Evolution and clinical impact of co-occurring genetic alterations in advanced-stage EGFR-mutant lung cancers. Nat Genet [Internet]. 2017;49(12):1693-1704. Disponible en: https://doi.org/10.1038/ng.3990
- Izumi H, Matsumoto S, Liu J, Tanaka K, Mori S, Hayashi K, et al. The CLIP1–LTK fusion is an oncogenic driver in non‐small‐cell lung cancer. Nature [Internet]. 2021;600(7888):319-323. Disponible en: https://doi.org/10.1038/s41586-021-04135-5
- Chen F, Huang C, Wu Q, Jiang L, Chen S, Chen L. Circular RNAs expression profiles in plasma exosomes from early-stage lung adenocarcinoma and the potential biomarkers. J Cell Biochem [Internet]. 2019;121(3):2525-2533. Disponible en: https://doi.org/10.1002/jcb.29475
- Zhu W, Zhang H, Tang L, Fang K, Lin N, Huang Y, et al. Identification of a Plasma Exosomal lncRNA- and circRNA-Based ceRNA Regulatory Network in Patients With Lung Adenocarcinoma. Clin Respir J [Internet]. 2024;18(10):e70026. Disponible en: https://doi.org/10.1111/crj.70026
- Lu S, Kato T, Dong X, Ahn MJ, Quang LV, Soparattanapaisarn N, et al. Osimertinib after Chemoradiotherapy in Stage III EGFR -Mutated NSCLC. N Engl J Med [Internet]. 2024;391(7):585-597. Disponible en: https://doi.org/10.1056/NEJMoa2402614
- Tsuboi M, Herbst RS, John T, Kato T, Majem M, Grohé C, et al. Overall Survival with Osimertinib in Resected EGFR -Mutated NSCLC. N Engl J Med [Internet]. 2023;389(2):137-147. Disponible en: https://doi.org/10.1056/NEJMoa2304594
- Wu YL, Dziadziuszko R, Ahn JS, Barlesi F, Nishio M, Lee DH, et al. Alectinib in Resected ALK -Positive Non–Small-Cell Lung Cancer. N Engl J Med [Internet]. 2024;390(14):1265-1276. Disponible en: https://doi.org/10.1056/NEJMoa2310532
- Chaft JE, Shyr Y, Sepesi B, Forde PM. Preoperative and Postoperative Systemic Therapy for Operable Non–Small-Cell Lung Cancer. J Clin Oncol [Internet]. 2022;40(6):546-555. Disponible en: https://doi.org/10.1200/jco.21.01589
- Herbst RS, John T, Grohé C, Goldman JW, Kato T, Laktionov K, et al. Molecular residual disease analysis of adjuvant osimertinib in resected EGFR-mutated stage IB–IIIA non-small-cell lung cancer. Nat Med [Internet]. 2025;31(6):1958-1968. Disponible en: https://doi.org/10.1038/s41591-025-03577-y
- Qin K, Hou H, Liang Y, Zhang X. Prognostic value of TP53 concurrent mutations for EGFR- TKIs and ALK-TKIs based targeted therapy in advanced non-small cell lung cancer: a meta-analysis. BMC Cancer [Internet]. 2020;20(1):328. Disponible en: https://doi.org/10.1186/s12885-020-06805-5
- Ricciuti B, Alessi JV, Elkrief A, Wang X, Cortellini A, Li YY, et al. Dissecting the clinicopathologic, genomic, and immunophenotypic correlates of KRASG12D-mutated non-small-cell lung cancer. Ann Oncol [Internet]. 2022;33(10):1029-1040. Disponible en: https://doi.org/10.1016/j.annonc.2022.07.005
- Peters S, Cho BC, Luft AV, Alatorre-Alexander J, Geater SL, Laktionov K, et al. Durvalumab With or Without Tremelimumab in Combination With Chemotherapy in First-Line Metastatic NSCLC: Five-Year Overall Survival Outcomes From the Phase 3 POSEIDON Trial. J Thorac Oncol [Internet]. 2025;20(1):76-93. Disponible en: https://doi.org/10.1016/j.jtho.2024.09.1381
- Ricciuti B, Wang X, Alessi JV, Rizvi H, Mahadevan NR, Li YY, et al. Association of High Tumor Mutation Burden in Non-Small Cell Lung Cancers with Increased Immune Infiltration and Improved Clinical Outcomes of PD-L1 Blockade Across PD-L1 Expression Levels. JAMA Oncol [Internet]. 2022;8(8):1160-1168. Disponible en: https://doi.org/10.1001/jamaoncol.2022.1981
- Johnson ML, Cho BC, Luft A, Alatorre-Alexander J, Geater SL, Laktionov K, et al. Durvalumab with or Without Tremelimumab in Combination with Chemotherapy as First-Line Therapy for Metastatic Non-Small-Cell Lung Cancer: The Phase III POSEIDON Study. J Clin Oncol [Internet]. 2023;41(6):1213-1227. Disponible en: https://doi.org/10.1200/JCO.22.00975
- Reck M, Ciuleanu TE, Cobo M, Schenker M, Zurawski B, Menezes J, et al. First-line nivolumab plus ipilimumab with two cycles of chemotherapy versus chemotherapy alone (four cycles) in advanced non-small-cell lung cancer: CheckMate 9LA 2-year update. ESMO Open [Internet]. 2021;6(5):100273. Disponible en: https://doi.org/10.1016/j.esmoop.2021.100273
- Niu D, Luo T, Wang H, Xia Y, Xie Z. Lactic acid in tumor invasion. Clin Chim Acta [Internet]. 2021;522:61-69. Disponible en: https://doi.org/10.1016/j.cca.2021.08.011
- Wang JX, Choi SYC, Niu X, Kang N, Xue H, Killam J, et al. Lactic acid and an acidic tumor microenvironment suppress anticancer immunity. Int J Mol Sci [Internet]. 2020;21(21):8363. Disponible en: https://doi.org/10.3390/ijms21218363
- Bo W, Wang X, Yu N, Wang C, Liu C. Shenqifuzheng injection inhibits lactic acid-induced cisplatin resistance in NSCLC by affecting FBXO22/p53 axis through FOXO3. Respir Res [Internet]. 2024;25(1):396. Disponible en: https://doi.org/10.1186/s12931-024-03013-8
- Gutiérrez-Sandoval R, Gutiérrez-Castro F, Muñoz-Godoy N, Rivadeneira I, Sobarzo A, Alarcón L, et al. The Design of a Multistage Monitoring Protocol for Dendritic Cell-Derived Exosome (DEX) Immunotherapy: A Conceptual Framework for Molecular Quality Control and Immune Profiling. Int J Mol Sci [Internet]. 2025;26(12):5444. Disponible en: https://doi.org/10.3390/ijms26125444
- Samadani AA, Keymoradzdeh A, Shams S, Soleymanpour A, Rashidy-Pour A, Hashemian H, et al. CAR T-cells profiling in carcinogenesis and tumorigenesis: An overview of CAR T-cells cancer therapy. Int Immunopharmacol [Internet]. 2021;90:107201. Disponible en: https://doi.org/10.1016/j.intimp.2020.107201
- Chan AML, Cheah JM, Lokanathan Y, Ng MH, Law JX. Natural Killer Cell-Derived Extracellular Vesicles as a Promising Immunotherapeutic Strategy for Cancer: A Systematic Review. Int J Mol Sci [Internet]. 2023;24(4):4026. Disponible en: https://doi.org/10.3390/ijms24044026
- Liu J, Feng Y, Zeng X, He M, Gong Y, Liu Y. Extracellular vesicles-encapsulated let-7i shed from bone mesenchymal stem cells suppress lung cancer via KDM3A/DCLK1/FXYD3 axis. J Cell Mol Med [Internet]. 2021;25(4):1911-1926. Disponible en: https://doi.org/10.1111/jcmm.15866
- Sun H, Zhu R, Guo X, Zhao P, Zhang R, Zhao Z, et al. Exosome miR-101–3p derived from bone marrow mesenchymal stem cells promotes radiotherapy sensitivity in non-small cell lung cancer by regulating DNA damage repair and autophagy levels through EZH2. Pathol Res Pract [Internet]. 2024;256:155237. Disponible en: https://doi.org/10.1016/j.prp.2024.155271
- Shokati A, Rahnama MA, Jalali L, Hoseinzadeh S, Masoudifar S, Ahmadvand M. Revolutionizing cancer treatment: engineering mesenchymal stem cell-derived small extracellular vesicles. Cancer Cell Int [Internet]. 2025;25(1):275. Disponible en: https://doi.org/10.1186/s12935-025-03900-0
- Chen YJ, Roumeliotis TI, Chang YH, Chen CT, Han CL, Lin MH, et al. Proteogenomics of Non-smoking Lung Cancer in East Asia Delineates Molecular Signatures of Pathogenesis and Progression. Cell [Internet]. 2020;182(1):226-244.e17. Disponible en: https://doi.org/10.1016/j.cell.2020.06.012
- Cho RJ, Alexandrov LB, den Breems NY, Atanasova VS, Farshchian M, Purdom E, et al. APOBEC mutation drives early-onset squamous cell carcinomas in recessive dystrophic epidermolysis bullosa. Sci Transl Med [Internet]. 2018;10(455):eaas9668. Disponible en: https://doi.org/10.1126/scitranslmed.aas9668
- Hellmann MD, Nathanson T, Rizvi H, Creelan BC, Sanchez-Vega F, Ahuja A, et al. Genomic Features of Response to Combination Immunotherapy in Patients with Advanced Non-Small-Cell Lung Cancer. Cancer Cell [Internet]. 2018;33(5):843-852.e4. Disponible en: https://doi.org/10.1016/j.ccell.2018.03.018
- Mori S, Izumi H, Araki M, Liu J, Tanaka Y, Kagawa Y, et al. LTK mutations responsible for resistance to lorlatinib in non-small cell lung cancer harboring CLIP1-LTK fusión. Commun Biol [Internet]. 2024;7(1):412. Disponible en: https://doi.org/10.1038/s42003-024-06116-6
- Cameron G, Nguyen T, Ciula M, Williams SJ, Godfrey DI. Glycolipids from the gut symbiont Bacteroides fragilis are agonists for natural killer T cells and induce their regulatory differentiation. Chem Sci [Internet]. 2023;14(29):7887-7896. Disponible en: https://doi.org/10.1039/d3sc02124f

















