Terapia CAR-T: Fundamentos moleculares, retos clínicos y nuevas perspectivas
CAR-T Therapy: Molecular Fundamentals, Clinical Challenges, and Emerging Perspectives
Cómo citar
Descargar cita

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-SinDerivadas 4.0.
Mostrar biografía de los autores
Introducción: la terapia con células T con receptor de antígeno quimérico (CAR-T) ha revolucionado el tratamiento de las neoplasias hematológicas, estableciendo un nuevo estándar de atención en la inmunoterapia celular personalizada. No obstante, pese a su notable éxito clínico, su aplicación generalizada continúa limitada por desafíos logísticos, biológicos y de seguridad.
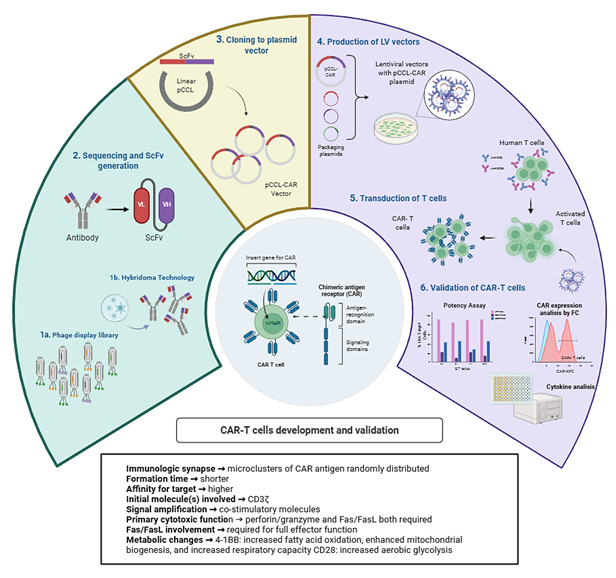
Métodos: en esta revisión se sintetizan los fundamentos moleculares de la biología de las células CAR-T a partir de evidencia preclínica y clínica. Se analizan los avances en el diseño de los receptores, la señalización coestimuladora y los procesos de fabricación, así como los mecanismos implicados en la resistencia terapéutica y la toxicidad.
Resultados: las mejoras iterativas en el diseño y la manufactura de CAR-T han incrementado significativamente la eficacia y la seguridad, conduciendo a múltiples aprobaciones de la FDA en leucemias de células B, linfomas y mieloma múltiple. Sin embargo, persisten limitaciones clave, incluyendo los largos tiempos de fabricación, el escape antigénico, el agotamiento de las células T, la persistencia limitada, la neurotoxicidad y la toxicidad on-target/off-tumor. Estrategias emergentes como la edición genómica, la generación de CAR-T alogénicas e in vivo, la reprogramación transcripcional y metabólica, los circuitos de biología sintética (SynNotch, SNIPR y CAR con compuertas lógicas), así como el descubrimiento de dianas conformacionales específicas y el uso de ligandos naturales, están mostrando un potencial prometedor para superar estas barreras.
Discusión: en conjunto, estos avances están transformando la terapia CAR-T en una plataforma más modular, programable y controlable, con mayor capacidad para abordar los mecanismos de resistencia y reducir la toxicidad asociada.
Conclusión: estas innovaciones anuncian una nueva era de inmunoterapias celulares más seguras y versátiles, con el potencial de expandir el impacto de la ingeniería de CAR más allá de las neoplasias hematológicas, incluyendo tumores sólidos y enfermedades inmunomediadas.
Visitas del artículo 0 | Visitas PDF 0
Descargas
- Zugasti I, Espinosa-Aroca, Lady, Fidyt K, Mulens-Arias V, Diaz-Beya M, Juan M, et al. CAR-T cell therapy for cancer: current challenges and future directions. Signal Transduct Target Ther [Internet]. 2025;10(1):210. Available from: https://doi.org/10.1038/s41392-025-02269-w
- Petri K, D’Ippolito E, Künkele A, Köhl U, Busch DH, Einsele H, et al. Next-generation T cell immunotherapies engineered with CRISPR base and prime editing: challenges and opportunities. Nat Rev Clin Oncol [Internet]. 2025;22(12):902–23. Available from: https://doi.org/10.1038/s41571-025-01072-4
- Singh N, Maus M V. Synthetic manipulation of the cancer-immunity cycle: CAR-T cell therapy. Immunity [Internet]. 2023;56(10):2296–310. Available from: https://doi.org/10.1016/j.immuni.2023.09.010
- June CH, O’Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science (1979) [Internet]. 2018;359(6382):1361–5. Available from: https://doi.org/10.1126/science.aar6711
- Mariuzza RA, Agnihotri P, Orban J. The structural basis of T-cell receptor (TCR) activation: An enduring enigma. Journal of Biological Chemistry [Internet]. 2020;295(4):914–25. Available from: https://doi.org/10.1016/s0021-9258(17)49904-2
- Kong S, Zhang J, Wang L, Li W, Guo H, Weng Q, et al. Mechanisms of low MHC I expression and strategies for targeting MHC I with small molecules in cancer immunotherapy. Cancer Lett [Internet]. 2025;611:217432. Available from: https://doi.org/10.1016/j.canlet.2024.217432
- Rodríguez-Nava C, Ortuño-Pineda C, Illades-Aguiar B, Flores-Alfaro E, Leyva-Vázquez MA, Parra-Rojas I, et al. Mechanisms of Action and Limitations of Monoclonal Antibodies and Single Chain Fragment Variable (scFv) in the Treatment of Cancer. Biomedicines [Internet]. 2023;11(6):1610. Available from: https://doi.org/10.3390/biomedicines11061610
- Teoh PJ, Chng WJ. CAR T-cell therapy in multiple myeloma: more room for improvement. Blood Cancer J [Internet]. 2021;11(4):84. Available from: https://doi.org/10.1038/s41408-021-00469-5
- Rafiq S, Hackett CS, Brentjens RJ. Engineering strategies to overcome the current roadblocks in CAR T cell therapy. Nat Rev Clin Oncol [Internet]. 2020;17(3):147–67. Available from: https://doi.org/10.1038/s41571-019-0297-y
- van der Heide V, Humblin E, Vaidya A, Kamphorst AO. Advancing beyond the twists and turns of T cell exhaustion in cancer. Sci Transl Med [Internet]. 2022;14(670). Available from: https://doi.org/10.1126/scitranslmed.abo4997
- Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. New England Journal of Medicine [Internet]. 2019;380(1):45–56. Available from: https://doi.org/10.1056/nejmoa1804980
- Locke FL, Miklos DB, Jacobson CA, Perales MA, Kersten MJ, Oluwole OO, et al. Axicabtagene Ciloleucel as Second-Line Therapy for Large B-Cell Lymphoma. New England Journal of Medicine [Internet]. 2022;386(7):640–54. Available from: https://doi.org/10.1056/nejmoa2116133
- Martin T, Usmani SZ, Berdeja JG, Agha M, Cohen AD, Hari P, et al. Ciltacabtagene Autoleucel, an Anti–B-cell Maturation Antigen Chimeric Antigen Receptor T-Cell Therapy, for Relapsed/Refractory Multiple Myeloma: CARTITUDE-1 2-Year Follow-Up. Journal of Clinical Oncology [Internet]. 2023;41(6):1265–74. Available from: https://doi.org/10.1200/JCO.22.00842
- Hong DS, Van Tine BA, Biswas S, McAlpine C, Johnson ML, Olszanski AJ, et al. Autologous T cell therapy for MAGE-A4+ solid cancers in HLA-A*02+ patients: a phase 1 trial. Nat Med [Internet]. 2023;29(1):104–14. Available from: https://doi.org/10.1038/s41591-022-02128-z
- Blache U, Popp G, Dünkel A, Koehl U, Fricke S. Potential solutions for manufacture of CAR T cells in cancer immunotherapy. Nat Commun [Internet]. 2022;13(1):5225. Available from: https://doi.org/10.1038/s41467-022-32866-0
- Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. New England Journal of Medicine [Internet]. 2018;378(5):439–48. Available from: https://doi.org/10.1056/nejmoa1709866
- Locke FL, Ghobadi A, Jacobson CA, Miklos DB, Lekakis LJ, Oluwole OO, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1–2 trial. Lancet Oncol [Internet]. 2019;20(1):31–42. Available from: https://doi.org/10.1016/S1470-2045(18)30864-7
- Abramson JS, Palomba ML, Gordon LI, Lunning MA, Wang M, Arnason J, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. The Lancet [Internet]. 2020;396(10254):839–52. Available from: https://doi.org/10.1016/s0140-6736(20)31366-0
- Wang M, Munoz J, Goy A, Locke FL, Jacobson CA, Hill BT, et al. KTE-X19 CAR T-Cell Therapy in Relapsed or Refractory Mantle-Cell Lymphoma. New England Journal of Medicine [Internet]. 2020;382(14):1331–42. Available from: https://doi.org/10.1056/nejmoa1914347
- Munshi NC, Anderson LD, Shah N, Madduri D, Berdeja J, Lonial S, et al. Idecabtagene Vicleucel in Relapsed and Refractory Multiple Myeloma. New England Journal of Medicine [Internet]. 2021;384(8):705–16. Available from: https://doi.org/10.1056/nejmoa2024850
- San-Miguel J, Dhakal B, Yong K, Spencer A, Anguille S, Mateos MV, et al. Cilta-cel or Standard Care in Lenalidomide-Refractory Multiple Myeloma. New England Journal of Medicine [Internet]. 2023;389(4):335–47. Available from: https://doi.org/10.1056/nejmoa2303379
- Berdeja JG, Madduri D, Usmani SZ, Jakubowiak A, Agha M, Cohen AD, et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. The Lancet [Internet]. 2021;398(10297):314–24. Available from: https://doi.org/10.1016/S0140-6736(21)00933-8
- Peng L, Sferruzza G, Yang L, Zhou L, Chen S. CAR-T and CAR-NK as cellular cancer immunotherapy for solid tumors. Cell Mol Immunol [Internet]. 2024 Aug 12;21(10):1089–108. Available from: https://doi.org/10.1038/s41423-024-01207-0
- Amini L, Silbert SK, Maude SL, Nastoupil LJ, Ramos CA, Brentjens RJ, et al. Preparing for CAR T cell therapy: patient selection, bridging therapies and lymphodepletion. Nat Rev Clin Oncol [Internet]. 2022;19(5):342–55. Available from: https://doi.org/10.1038/s41571-022-00607-3
- Hu B, Vaidya R, Ahmed F, Ehsan H, Moyo TK, Jacobs RW, et al. Real-World Analysis of Barriers to Timely Administration of Chimeric Antigen Receptor T Cell (CAR T) Therapy in Diffuse Large B-cell Lymphoma. Transplant Cell Ther [Internet]. 2024 Nov 1;30(11):1082.e1-1082.e10. Available from: https://doi.org/10.1016/j.jtct.2024.09.007
- Lei T, Wang Y, Zhang Y, Yang Y, Cao J, Huang J, et al. Leveraging CRISPR gene editing technology to optimize the efficacy, safety and accessibility of CAR T-cell therapy. Leukemia [Internet]. 2024;38(12):2517–43. Available from: https://doi.org/10.1038/s41375-024-02444-y
- Kamali E, Rahbarizadeh F, Hojati Z, Frödin M. CRISPR/Cas9-mediated knockout of clinically relevant alloantigenes in human primary T cells. BMC Biotechnol [Internet]. 2021;21(1):9. Available from: https://doi.org/10.1186/s12896-020-00665-4
- Georgiadis C, Preece R, Qasim W. Clinical development of allogeneic chimeric antigen receptor αβ-T cells. Molecular Therapy [Internet]. 2025;33(6):2426–40. Available from: https://doi.org/10.1016/j.ymthe.2025.03.040
- Guo S, Lei W, Jin X, Liu H, Wang JQ, Deng W, et al. CD70-specific CAR NK cells expressing IL-15 for the treatment of CD19-negative B-cell malignancy. Blood Adv [Internet]. 2024;8(11):2635–45. Available from: https://doi.org/10.1182/bloodadvances.2023012202
- Liu E, Marin D, Banerjee P, Macapinlac HA, Thompson P, Basar R, et al. Use of CAR-Transduced Natural Killer Cells in CD19-Positive Lymphoid Tumors. New England Journal of Medicine [Internet]. 2020;382(6):545–53. Available from: https://doi.org/10.1056/nejmoa1910607
- Bot A, Scharenberg A, Friedman K, Guey L, Hofmeister R, Andorko JI, et al. In vivo chimeric antigen receptor (CAR)-T cell therapy. Nat Rev Drug Discov [Internet]. 2025. Available from: https://doi.org/10.1038/s41573-025-01291-5
- Nyberg WA, Wang CH, Ark J, Liu C, Clouden S, Qualls A, et al. In vivo engineering of murine T cells using the evolved adeno-associated virus variant Ark313. Immunity [Internet]. 2025;58(2):499-512.e7. Available from: https://doi.org/10.1016/j.immuni.2025.01.009
- Hamilton JR, Chen E, Perez BS, Sandoval Espinoza CR, Kang MH, Trinidad M, et al. In vivo human T cell engineering with enveloped delivery vehicles. Nat Biotechnol [Internet]. 2024;42(11):1684–92. Available from: https://doi.org/10.1038/s41587-023-02085-z
- Xu J, Liu L, Parone P, Xie W, Sun C, Chen Z, et al. In-vivo B-cell maturation antigen CAR T-cell therapy for relapsed or refractory multiple myeloma. The Lancet [Internet]. 2025;406(10500):228–31. Available from: https://doi.org/10.1016/s0140-6736(25)01030-x
- Long AH, Haso WM, Shern JF, Wanhainen KM, Murgai M, Ingaramo M, et al. 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat Med [Internet]. 2015;21(6):581–90. Available from: https://doi.org/10.1038/nm.3838
- Lynn RC, Weber EW, Sotillo E, Gennert D, Xu P, Good Z, et al. c-Jun overexpression in CAR T cells induces exhaustion resistance. Nature [Internet]. 2019;576(7786):293–300. Available from: https://doi.org/10.1038/s41586-019-1805-z
- Kumar B, Singh A, Basar R, Uprety N, Li Y, Fan H, et al. BATF is a major driver of NK cell epigenetic reprogramming and dysfunction in AML. Sci Transl Med [Internet]. 2024;16(764):4. Available from: https://doi.org/10.1126/scitranslmed.adp0004
- Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol [Internet]. 2015;15(8):486–99. Available from: https://doi.org/10.1038/nri3862
- Philipson BI, O’Connor RS, May MJ, June CH, Albelda SM, Milone MC. 4-1BB costimulation promotes CAR T cell survival through noncanonical NF-κB signaling. Sci Signal [Internet]. 2020;13(625):31. Available from: https://doi.org/10.1126/scisignal.aay8248
- Roselli E, Boucher JC, Li G, Kotani H, Spitler K, Reid K, et al. 4-1BB and optimized CD28 co-stimulation enhances function of human mono-specific and bi-specific third-generation CAR T cells. J Immunother Cancer [Internet]. 2021;9(10):e003354. Available from: https://doi.org/10.1136/jitc-2021-003354
- Roselli E, Boucher JC, Li G, Kotani H, Spitler K, Reid K, et al. 4-1BB and optimized CD28 co-stimulation enhances function of human mono-specific and bi-specific third-generation CAR T cells. J Immunother Cancer. 2021;9(10): 9:e003354. Available from: https://doi.org/10.1136/jitc-2021-003354
- Wijewarnasuriya D, Bebernitz C, Lopez A V., Rafiq S, Brentjens RJ. Excessive Costimulation Leads to Dysfunction of Adoptively Transferred T Cells. Cancer Immunol Res [Internet]. 2020;8(6):732–42. Available from: https://doi.org/10.1158/2326-6066.CIR-19-0908
- Liu C, Qi T, Milner JJ, Lu Y, Cao Y. Speed and Location Both Matter: Antigen Stimulus Dynamics Controls CAR-T Cell Response. Front Immunol [Internet]. 2021;12. Available from: https://doi.org/10.3389/fimmu.2021.748768
- Chen J, Qiu S, Li W, Wang K, Zhang Y, Yang H, et al. Tuning charge density of chimeric antigen receptor optimizes tonic signaling and CAR-T cell fitness. Cell Res [Internet]. 2023;33(5):341–54. Available from: https://doi.org/10.1038/s41422-023-00789-0
- Davenport AJ, Cross RS, Watson KA, Liao Y, Shi W, Prince HM, et al. Chimeric antigen receptor T cells form nonclassical and potent immune synapses driving rapid cytotoxicity. Proceedings of the National Academy of Sciences [Internet]. 2018 Feb 27;115(9):E2068–76. Available from: https://doi.org/10.1073/pnas.1716266115
- Majzner RG, Rietberg SP, Sotillo E, Dong R, Vachharajani VT, Labanieh L, et al. Tuning the Antigen Density Requirement for CAR T-cell Activity. Cancer Discov [Internet]. 2020;10(5):702–23. Available from: https://doi.org/10.1158/2159-8290.CD-19-0945
- Maher J, Brentjens RJ, Gunset G, Rivière I, Sadelain M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRζ /CD28 receptor. Nat Biotechnol [Internet]. 2002;20(1):70–5. Available from: https://doi.org/10.1038/nbt0102-70
- Feucht J, Sun J, Eyquem J, Ho YJ, Zhao Z, Leibold J, et al. Calibration of CAR activation potential directs alternative T cell fates and therapeutic potency. Nat Med [Internet]. 2019;25(1):82–8. Available from: https://doi.org/10.1038/s41591-018-0290-5
- Man K, Gabriel SS, Liao Y, Gloury R, Preston S, Henstridge DC, et al. Transcription Factor IRF4 Promotes CD8+ T Cell Exhaustion and Limits the Development of Memory-like T Cells during Chronic Infection. Immunity [Internet]. 2017;47(6):1129-1141.e5. Available from: https://doi.org/10.1016/j.immuni.2017.11.021
- Li P, Spolski R, Liao W, Wang L, Murphy TL, Murphy KM, et al. BATF–JUN is critical for IRF4-mediated transcription in T cells. Nature [Internet]. 2012;490(7421):543–6. Available from: https://doi.org/10.1038/nature11530
- Poorebrahim M, Melief J, Pico de Coaña Y, L. Wickström S, Cid-Arregui A, Kiessling R. Counteracting CAR T cell dysfunction. Oncogene [Internet]. 2021 Jan 14;40(2):421–35. Available from: https://doi.org/10.1038/s41388-020-01501-x
- Seo H, Chen J, González-Avalos E, Samaniego-Castruita D, Das A, Wang YH, et al. TOX and TOX2 transcription factors cooperate with NR4A transcription factors to impose CD8 + T cell exhaustion. Proceedings of the National Academy of Sciences [Internet]. 2019;116(25):12410–5. Available from: https://doi.org/10.1073/pnas.1905675116
- John LB, Devaud C, Duong CPM, Yong CS, Beavis PA, Haynes NM, et al. Anti-PD-1 Antibody Therapy Potently Enhances the Eradication of Established Tumors By Gene-Modified T Cells. Clinical Cancer Research [Internet]. 2013;19(20):5636–46. Available from: https://doi.org/10.1158/1078-0432.ccr-13-0458
- Ouyang W, Jin SW, Xu N, Liu WY, Zhao H, Zhang L, et al. PD-1 downregulation enhances CAR-T cell antitumor efficiency by preserving a cell memory phenotype and reducing exhaustion. J Immunother Cancer [Internet]. 2024;12(4):e008429. Available from: https://doi.org/10.1136/jitc-2023-008429
- Gumber D, Wang LD. Improving CAR-T immunotherapy: Overcoming the challenges of T cell exhaustion. EBioMedicine [Internet]. 2022;77:103941. Available from: https://doi.org/10.1016/j.ebiom.2022.103941
- Gabriel SS, Tsui C, Chisanga D, Weber F, Llano-León M, Gubser PM, et al. Transforming growth factor-β-regulated mTOR activity preserves cellular metabolism to maintain long-term T cell responses in chronic infection. Immunity [Internet]. 2021;54(8):1698-1714.e5. Available from: https://doi.org/10.1016/j.immuni.2021.06.007
- Mestermann K, Giavridis T, Weber J, Rydzek J, Frenz S, Nerreter T, et al. The tyrosine kinase inhibitor dasatinib acts as a pharmacologic on/off switch for CAR T cells. Sci Transl Med [Internet]. 2019;11(499):5907. Available from: https://doi.org/10.1126/scitranslmed.aau5907
- Morris EC, Neelapu SS, Giavridis T, Sadelain M. Cytokine release syndrome and associated neurotoxicity in cancer immunotherapy. Nat Rev Immunol [Internet]. 2022;22(2):85–96. Available from: https://doi.org/10.1038/s41577-021-00547-6
- Mulvey A, Trueb L, Coukos G, Arber C. Novel strategies to manage CAR-T cell toxicity. Nat Rev Drug Discov [Internet]. 2025;24(5):379–97. Available from: https://doi.org/10.1038/s41573-024-01100-5
- Garcia J, Daniels J, Lee Y, Zhu I, Cheng K, Liu Q, et al. Naturally occurring T cell mutations enhance engineered T cell therapies. Nature [Internet]. 2024;626(7999):626–34. Available from: https://doi.org/10.1038/s41586-024-07018-7
- Flugel CL, Majzner RG, Krenciute G, Dotti G, Riddell SR, Wagner DL, et al. Overcoming on-target, off-tumour toxicity of CAR T cell therapy for solid tumours. Nat Rev Clin Oncol [Internet]. 2023;20(1):49–62. Available from: https://doi.org/10.1038/s41571-022-00704-3
- Monje M, Mahdi J, Majzner R, Yeom KW, Schultz LM, Richards RM, et al. Intravenous and intracranial GD2-CAR T cells for H3K27M+ diffuse midline gliomas. Nature [Internet]. 2025;637(8046):708–15. Available from: https://doi.org/10.1038/s41586-024-08171-9
- Shabaneh TB, Stevens AR, Stull SM, Shimp KR, Seaton BW, Gad EA, et al. Systemically administered low-affinity HER2 CAR T cells mediate antitumor efficacy without toxicity. J Immunother Cancer [Internet]. 2024;12(2):e008566. Available from: https://doi.org/10.1136/jitc-2023-008566
- Anderson J, Barone G, Zehner A. GD2 targeting CAR T cells for neuroblastoma. EJC Paediatric Oncology [Internet]. 2024 Dec 1;4:100179. Available from: https://doi.org/10.1016/j.ejcped.2024.100179
- Zhu L, Liu J, Li J, Wang N, Zhao Y, Su H. Research progress on HER2-specific chimeric antigen receptor T cells for immunotherapy of solid tumors. Front Immunol [Internet]. 2025;16. Available from: https://doi.org/10.3389/fimmu.2025.1514994
- Garfall AL, Cohen AD, Susanibar-Adaniya SP, Hwang WT, Vogl DT, Waxman AJ, et al. Anti-BCMA/CD19 CAR T Cells with Early Immunomodulatory Maintenance for Multiple Myeloma Responding to Initial or Later-Line Therapy. Blood Cancer Discov [Internet]. 2023;4(2):118–33. Available from: https://doi.org/10.1158/2643-3230.BCD-22-0074
- Shi M, Wang J, Huang H, Liu D, Cheng H, Wang X, et al. Bispecific CAR T cell therapy targeting BCMA and CD19 in relapsed/refractory multiple myeloma: a phase I/II trial. Nat Commun [Internet]. 2024;15(1):3371. Available from: https://doi.org/10.1038/s41467-024-47801-8
- Cappell KM, Kochenderfer JN. Long-term outcomes following CAR T cell therapy: what we know so far. Vol. 20, Nature Reviews Clinical Oncology. Springer Nature; [Internet]. 2023. p. 359–71. Available from: https://doi.org/10.1038/s41571-023-00754-1
- Wang Y, Li C, Xia J, Li P, Cao J, Pan B, et al. Humoral immune reconstitution after anti-BCMA CAR T-cell therapy in relapsed/refractory multiple myeloma. Blood Adv [Internet]. 2021;5(23):5290–9. Available from: https://doi.org/10.1182/bloodadvances.2021004603
- Jiang Y li, Li Q, Yuan T, Jiang Y yu, Deng Q.
Case Report of Anti-CD123 Chimeric Antigen Receptor T-Cell Therapy Followed by Radiotherapy for a Recurrence of Blastic Plasmacytoid Dendritic Cell Neoplasm After Allogeneic Hematopoietic Stem Cell Transplantation
. Onco Targets Ther [Internet]. 2020 Apr;Volume 13:3425–30. Available from: https://doi.org/10.2147/ott.s250016 - Perna F, Parekh S, Diorio C, Smith M, Subklewe M, Mehta R, et al. CAR T-cell toxicities: from bedside to bench, how novel toxicities inform laboratory investigations. Blood Adv [Internet]. 2024;8(16):4348–58. Available from: https://doi.org/10.1182/bloodadvances.2024013044
- Thompson JA, Schneider BJ, Brahmer J, Zaid MA, Achufusi A, Armand P, et al. NCCN Guidelines® Insights: Management of Immunotherapy-Related Toxicities, Version 2.2024. Journal of the National Comprehensive Cancer Network [Internet]. 2024;22(9):582–92. Available from: https://doi.org/10.6004/jnccn.2024.0057
- Kumar AD, Atallah-Yunes SA, Rajeeve S, Abdelhak A, Hashmi H, Corraes A, et al. Delayed Neurotoxicity after CAR-T in Multiple Myeloma: Results from a Global IMWG Registry. Blood [Internet]. 2024;144(Supplement 1):4758–4758. Available from: https://doi.org/10.1182/blood-2024-201783
- Parker KR, Migliorini D, Perkey E, Yost KE, Bhaduri A, Bagga P, et al. Single-Cell Analyses Identify Brain Mural Cells Expressing CD19 as Potential Off-Tumor Targets for CAR-T Immunotherapies. Cell [Internet]. 2020;183(1):126-142.e17. Available from: https://doi.org/10.1016/j.cell.2020.08.022
- Ferguson ID, Patiño-Escobar B, Tuomivaara ST, Lin YHT, Nix MA, Leung KK, et al. The surfaceome of multiple myeloma cells suggests potential immunotherapeutic strategies and protein markers of drug resistance. Nat Commun [Internet]. 2022;13(1):4121. Available from: https://doi.org/10.1038/s41467-022-31810-6
- Patiño-Escobar B, Perez-Lugo L, Wiita AP. Technology Spotlight Surface Target Discovery for Blood Cancer Immunotherapies. The Hematologist [Internet]. 2025;22(1). Available from: https://doi.org/10.1182/hem.v22.1.2025214
- Mandal K, Wicaksono G, Yu C, Adams JJ, Hoopmann MR, Temple WC, et al. Structural surfaceomics reveals an AML-specific conformation of integrin β2 as a CAR T cellular therapy target. Nat Cancer [Internet]. 2023;4(11):1592–609. Available from: https://doi.org/10.1101/2022.10.10.511511
- Kloss CC, Condomines M, Cartellieri M, Bachmann M, Sadelain M. Combinatorial antigen recognition with balanced signaling promotes selective tumor eradication by engineered T cells. Nat Biotechnol [Internet]. 2013;31(1):71–5. Available from: https://doi.org/10.1038/nbt.2459
- Zhang W, He Q, Lopez B, Hu J, Kundu A, Andraza MC, et al. Abstract PO074: Logic-gating HER2 CAR-T to the tumor microenvironment mitigates on-target, off-tumor toxicity without compromising cytotoxicity against HER2-over-expressing tumors. Cancer Immunol Res [Internet]. 2021;9(2_Supplement):PO074–PO074. Available from: https://doi.org/10.1158/2326-6074.tumimm20-po074
- Juillerat A, Marechal A, Filhol JM, Valogne Y, Valton J, Duclert A, et al. An oxygen sensitive self-decision making engineered CAR T-cell. Sci Rep [Internet]. 2017;7(1):39833. Available from: https://doi.org/10.1038/srep39833
- Roybal KT, Williams JZ, Morsut L, Rupp LJ, Kolinko I, Choe JH, et al. Engineering T Cells with Customized Therapeutic Response Programs Using Synthetic Notch Receptors. Cell [Internet]. 2016;167(2):419-432.e16. Available from: https://doi.org/10.1016/j.cell.2016.09.011
- Zhu I, Liu R, Garcia JM, Hyrenius-Wittsten A, Piraner DI, Alavi J, et al. Modular design of synthetic receptors for programmed gene regulation in cell therapies. Cell [Internet]. 2022;185(8):1431-1443.e16. Available from: https://doi.org/10.1016/j.cell.2022.03.023
- Garcia JM, Burnett CE, Roybal KT. Toward the clinical development of synthetic immunity to cancer. Immunological Reviews. John Wiley and Sons Inc; [Internet]. 2023. Available from: https://doi.org/10.1111/imr.13245
- Lin H, Yang X, Ye S, Huang L, Mu W. Antigen escape in CAR-T cell therapy: Mechanisms and overcoming strategies. Biomedicine & Pharmacotherapy [Internet]. 2024;178:117252. Available from: https://doi.org/10.1016/j.biopha.2024.117252
- Lu Y, Zhao F. Strategies to overcome tumour relapse caused by antigen escape after CAR T therapy. Mol Cancer [Internet]. 2025;24(1):126. Available from: https://doi.org/10.1186/s12943-025-02334-6
- Samur MK, Fulciniti M, Aktas Samur A, Bazarbachi AH, Tai YT, Prabhala R, et al. Biallelic loss of BCMA as a resistance mechanism to CAR T cell therapy in a patient with multiple myeloma. Nat Commun [Internet]. 2021;12(1):868. Available from: https://doi.org/10.1182/bloodadvances.2023010025
- Samur MK, Aktas Samur A, Corre J, Lannes R, Shah P, Anderson K, et al. Monoallelic deletion of BCMA is a frequent feature in multiple myeloma. Blood Adv [Internet]. 2023;7(21):6599–603. Available from: https://doi.org/10.1182/bloodadvances.2023010025
- Cordoba S, Onuoha S, Thomas S, Pignataro DS, Hough R, Ghorashian S, et al. CAR T cells with dual targeting of CD19 and CD22 in pediatric and young adult patients with relapsed or refractory B cell acute lymphoblastic leukemia: a phase 1 trial. Nat Med [Internet]. 2021;27(10):1797–805. Available from: https://doi.org/10.1038/s41591-021-01497-1
- Spiegel JY, Patel S, Muffly L, Hossain NM, Oak J, Baird JH, et al. CAR T cells with dual targeting of CD19 and CD22 in adult patients with recurrent or refractory B cell malignancies: a phase 1 trial. Nat Med [Internet]. 2021;27(8):1419–31. Available from: https://doi.org/10.1038/s41591-021-01436-0
- Simon S, Riddell SR. Dual Targeting with CAR T Cells to Limit Antigen Escape in Multiple Myeloma. Blood Cancer Discov [Internet]. 2020;1(2):130–3. Available from: https://doi.org/10.1158/2643-3230.bcd-20-0122
- Zhou D, Sun Q, Xia J, Gu W, Qian J, Zhuang W, et al. Anti-BCMA/GPRC5D bispecific CAR T cells in patients with relapsed or refractory multiple myeloma: a single-arm, single-centre, phase 1 trial. Lancet Haematol [Internet]. 2024;11(10):e751–60. Available from: https://doi.org/10.1016/s2352-3026(24)00176-5
- Mailankody S, Devlin SM, Landa J, Nath K, Diamonte C, Carstens EJ, et al. GPRC5D-Targeted CAR T Cells for Myeloma. New England Journal of Medicine [Internet]. 2022;387(13):1196–206. Available from: https://doi.org/10.1056/nejmoa2209900
- Tong C, Zhang Y, Liu Y, Ji X, Zhang W, Guo Y, et al. Optimized tandem CD19/CD20 CAR-engineered T cells in refractory/relapsed B-cell lymphoma [Internet]. 2020. Available from: https://doi.org/10.1182/blood.2022017502
- Hegde M, Mukherjee M, Grada Z, Pignata A, Landi D, Navai SA, et al. Tandem CAR T cells targeting HER2 and IL13Rα2 mitigate tumor antigen escape. Journal of Clinical Investigation [Internet]. 2016;126(8):3036–52. Available from: https://doi.org/10.1172/jci83416
- Fedorov VD, Themeli M, Sadelain M. PD-1– and CTLA-4–Based Inhibitory Chimeric Antigen Receptors (iCARs) Divert Off-Target Immunotherapy Responses. Sci Transl Med [Internet]. 2013;5(215). Available from: https://doi.org/10.1126/scitranslmed.3006597
- FDA Requires Boxed Warning for T cell Malignancies Following Treatment with BCMA-Directed or CD19-Directed Autologous Chimeric Antigen Receptor (CAR) T cell Immunotherapies [Internet]. FDA. 2024. Available from: https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/fda-requires-boxed-warning-t-cell-malignancies-following-treatment-bcma-directed-or-cd19-directed
- Prasad V. T-Cell Lymphoma From CAR T-Cell Therapy—A New Safety Notice. JAMA [Internet]. 2024;331(5):389. Available from: https://doi.org/10.1001/jama.2023.27885
- Hamilton MP, Sugio T, Noordenbos T, Shi S, Bulterys PL, Liu CL, et al. Risk of Second Tumors and T-Cell Lymphoma after CAR T-Cell Therapy. New England Journal of Medicine [Internet]. 2024;390(22):2047–60. Available from: https://doi.org/10.1056/nejmoa2401361
- Perica K, Jain N, Scordo M, Patel R, Eren OC, Patel U, et al. CD4+ T-Cell Lymphoma Harboring a Chimeric Antigen Receptor Integration in TP53. New England Journal of Medicine [Internet]. 2025;392(6):577–83. Available from: https://doi.org/10.1056/nejmoa2411507
- Aleman A, Van Oekelen O, Melnekoff DT, Grossman L, Mouhieddine TH, Kurowski A, et al. Targeted Therapy of CAR+ T-Cell Lymphoma after Anti-BCMA CAR T-Cell Therapy. New England Journal of Medicine [Internet]. 2025 Aug 21;393(8):823–5. Available from: https://doi.org/10.1056/NEJMc2504588
- Gehrke L, Gonçalves VDR, Andrae D, Rasko T, Ho P, Einsele H, et al. Current Non-Viral-Based Strategies to Manufacture CAR-T Cells. Int J Mol Sci [Internet]. 2024;25(24):13685. Available from: https://doi.org/10.3390/ijms252413685
- Eyquem J, Mansilla-Soto J, Giavridis T, van der Stegen SJC, Hamieh M, Cunanan KM, et al. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature [Internet]. 2017;543(7643):113–7. Available from: https://doi.org/10.1038/nature21405
- Mansilla-Soto J, Eyquem J, Haubner S, Hamieh M, Feucht J, Paillon N, et al. HLA-independent T cell receptors for targeting tumors with low antigen density. Nat Med [Internet]. 2022;28(2):345–52. Available from: https://doi.org/10.1038/s41591-021-01621-1
- Moghanloo E, Mollanoori H, Talebi M, Pashangzadeh S, Faraji F, Hadjilooei F, et al. Remote controlling of CAR-T cells and toxicity management: Molecular switches and next generation CARs. Transl Oncol [Internet]. 2021;14(6):101070. Available from: https://doi.org/10.1016/j.tranon.2021.101070
- D’Aloia MM, Zizzari IG, Sacchetti B, Pierelli L, Alimandi M. CAR-T cells: the long and winding road to solid tumors. Cell Death Dis [Internet]. 2018;9(3):282. Available from: https://doi.org/10.1038/s41419-018-0278-6
- Gattinoni L, Speiser DE, Lichterfeld M, Bonini C. T memory stem cells in health and disease. Nat Med [Internet]. 2017;23(1):18–27. Available from: https://doi.org/10.1038/nm.4241
- Xu Y, Zhang M, Ramos CA, Durett A, Liu E, Dakhova O, et al. Closely related T-memory stem cells correlate with in vivo expansion of CAR.CD19-T cells and are preserved by IL-7 and IL-15. Blood. [Internet]. 2014;123(24):3750–9. Available from: https://doi.org/10.1182/blood-2014-01-552174
- Torres Chavez AG, McKenna MK, Gupta A, Daga N, Vera J, Leen AM, et al. IL-7 armed binary CAR T cell strategy to augment potency against solid tumors. Front Immunol [Internet]. 2025;16. Available from: https://doi.org/10.3389/fimmu.2025.1618404
- Shen Z, Sang Z, Shi Y. Nanobodies as a powerful platform for biomedicine. Trends Mol Med [Internet]. 2022 Nov 1;28(11):1006–7. Available from: https://doi.org/10.1016/j.molmed.2022.08.007
- Nix MA, Mandal K, Geng H, Paranjape N, Lin YHT, Rivera JM, et al. Surface Proteomics Reveals CD72 as a Target for In Vitro –Evolved Nanobody-Based CAR-T Cells in KMT2A/MLL1 -Rearranged B-ALL. Cancer Discov [Internet]. 2021;11(8):2032–49. Available from: https://doi.org/10.1158/2159-8290.CD-20-0242
- Schmidts A, Ormhøj M, Choi BD, Taylor AO, Bouffard AA, Scarfò I, et al. Rational design of a trimeric APRIL-based CAR-binding domain enables efficient targeting of multiple myeloma. Blood Adv [Internet]. 2019;3(21):3248–60. Available from: https://doi.org/10.1182/bloodadvances.2019000703
- Kasap C, Izgutdina A, Patiño-Escobar B, Kang AS, Chilakapati N, Akagi N, et al. Targeting high-risk multiple myeloma genotypes with optimized anti-CD70 CAR T cells. Blood [Internet]. 2025;146(7):819–33. Available from: https://doi.org/10.1182/blood.2024025536
- Grauwet K, Berger T, Kann MC, Silva H, Larson R, Leick MB, et al. Stealth transgenes enable CAR-T cells to evade host immune responses. J Immunother Cancer [Internet]. 2024;12(5):e008417. Available from: https://doi.org/10.1136/jitc-2023-008417
- Patino-Escobar B, Kasap C, Ferguson I, Hale M, Wiita A. P-103: Profiling the myeloma cell surface proteome reveals CCR10 as a potential immunotherapeutic target. Clin Lymphoma Myeloma Leuk [Internet]. 2021;21:S94–5. Available from: https://doi.org/10.1016/s2152-2650(21)02237-0
- Chilakapati N, Patiño-Escobar B, Chen EY, Dalal R, de Montagnac J, Johnson H, et al. Structure-guided engineering of CCL27 enhances natural ligand CAR T-cells against CCR10 for multiple myeloma [Internet]. 2025. Available from: https://doi.org/10.1101/2025.08.19.670119
- Chen AXY, Yap KM, Kim JS, Sek K, Huang YK, Dunbar PA, et al. Rewiring endogenous genes in CAR T cells for tumour-restricted payload delivery. Nature [Internet]. 2025;644(8075):241–51. Available from: https://doi.org/10.1038/s41586-025-09212-7
- Edelstein HI, Cosio A, Ezekiel ML, Corcoran WK, Morris AH, Leonard JN. Conversion of natural cytokine receptors into orthogonal synthetic biosensors. Nat Chem Biol [Internet]. 2025;21(11):1719–30. Available from: https://doi.org/10.1101/2024.03.23.586421

















