Patología molecular y biobancos en medicina de precisión del cáncer
Molecular pathology and biobanks in precision cancer medicine
Cómo citar
Descargar cita

Esta obra está bajo una licencia internacional Creative Commons Atribución-NoComercial-SinDerivadas 4.0.
Mostrar biografía de los autores
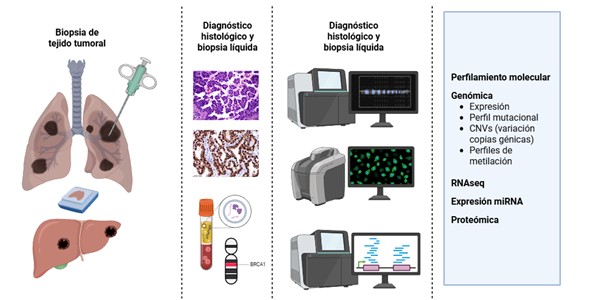
Introducción: la patología molecular constituye un eje central de la medicina de precisión en oncología, al integrar hallazgos morfológicos, inmunohistoquímicos y técnicas de biología molecular para identificar biomarcadores en ADN, ARN y proteínas. Estos biomarcadores impactan de manera directa en el diagnóstico, el pronóstico y la selección de terapias personalizadas.
Métodos: Se realizó una revisión de la literatura en bases de datos como PubMed, Embase, Scopus, y Google Scholar.
Resultados: entre las técnicas más empleadas se incluyen la inmunohistoquímica, la hibridación in situ, la reacción en cadena de la polimerasa y la secuenciación de nueva generación (NGS). Dichas metodologías permiten detectar alteraciones genómicas como variantes puntuales, indeles, variaciones en el número de copias y fusiones, con consecuencias relevantes en la evolución tumoral y la respuesta terapéutica.
Discusión: actualmente, se dispone de una amplia gama de biomarcadores en los cuales existe terapía dirigida basado en la alteración molecular puntual. De igual forma, han cobrado importancia los biomarcadores agnósticos que permiten decisiones terapéuticas transversales, independientemente del origen histológico. En paralelo, la evaluación de PD-L1, se ha convertido en una herramienta esencial para seleccionar pacientes candidatos a inmunoterapia. En este escenario de la patología molecular, los biobancos emergen como infraestructuras estratégicas para la medicina de precisión, al garantizar trazabilidad, calidad y estandarización en la gestión de muestras y datos asociados.
Conclusión: el Biobanco Nacional de Tumores Terry Fox, en Colombia, representa un ejemplo regional de cómo estas plataformas fortalecen la investigación traslacional y promueven colaboraciones internacionales.
Visitas del artículo 0 | Visitas PDF 0
Descargas
- Parra-Medina R. Patología oncológica molecular aplicada. Primera edición. Fundación Universitaria de Ciencias de la Salud; 2024.
- da Cunha IW, de Almeida Coudry R, de Macedo MP, de Assis EACP, Stefani S, Soares FA. A call to action: molecular pathology in Brazil. Surgical and Experimental Pathology. [Internet] 202;4(1):15. Disponible en: https://doi.org/10.1186/s42047-021-00096-1
- Chen H, Luthra R, Goswami RS, Singh RR, Roy-Chowdhuri S. Analysis of Pre-Analytic Factors Affecting the Success of Clinical Next-Generation Sequencing of Solid Organ Malignancies. Cancers (Basel). [Internet] 2015;7(3):1699–715. Disponible en: https://doi.org/10.3390/cancers7030859.
- Tjota MY, Segal JP, Wang P. Clinical Utility and Benefits of Comprehensive Genomic Profiling in Cancer. J Appl Lab Med. [Internet] 2024;9(1):76–91. Disponible en: https://doi.org/10.1093/jalm/jfad091.
- Spencer S, Ye W, Peng S, Zou D. Cost-Effectiveness Analysis of Comprehensive Genomic Profiling in Patients with Advanced Non-Small-Cell Lung Cancer Using Real-World Data. J Mol Diagn. [Internet] 2025;27(9):850–8. Disponible en: https://doi.org/10.1016/j.jmoldx.2025.05.011.
- Aldea M, Friboulet L, Apcher S, Jaulin F, Mosele F, Sourisseau T, et al. Precision medicine in the era of multi-omics: can the data tsunami guide rational treatment decision? ESMO Open. [Internet] 2023;8(5):101642. Disponible en: https://doi.org/10.1016/j.esmoop.2023.101642.
- Saldanha EF, Cordeiro de Lima VC, Fares A, Corassa M, Gil-Santana L, Arrieta O, et al. Real-world characteristics and outcomes of ERBB2-mutant NSCLC in Latin American patients (CLICaP). Oncologist. [Internet] 2025;30(2). Disponible en: https://doi.org/10.1093/oncolo/oyae347.
- Deininger M, Buchdunger E, Druker BJ. The development of imatinib as a therapeutic agent for chronic myeloid leukemia. Blood. [Internet] 2005;105(7):2640–53. Disponible en: https://doi.org/10.1182/blood-2004-08-3097.
- Jørgensen JT. Twenty-five years with companion diagnostics. Chin Clin Oncol. [Internet] 2023;12(6):65–65. Disponible en: https://doi.org/10.21037/cco-23-96.
- Mendivelso-González DF, Clavijo Cabezas D, Montoya L, Plazas Vargas M, López-Correa P, Colón E, et al. HER2-low prevalence among Hispanic/Latino women with breast cancer: A systematic review and meta-analysis. PLoS One. [Internet] 2024;19(12):e0315287. Disponible en: https://doi.org/10.1371/journal.pone.0315287.
- Sholl LM, Cooper WA, Kerr KM, Tan D, Tsao MS et al. (Eds). IASLC Atlas of molecular testing for targeted therapy in lung cancer. Denver: International Association for the Study of Lung Cancer; [Internet] 2023. Disponible en: https://www.iaslc.org/research-education/publications-resources-guidelines/iaslc-atlas-molecular-testing-targeting-2
- Parra-Medina R, Castañeda-González JP, Montoya L, Paula Gómez-Gómez M, Clavijo Cabezas D, Plazas Vargas M. Prevalence of oncogenic driver mutations in Hispanics/Latin patients with lung cancer. A systematic review and meta-analysis. Lung Cancer. [Internet] 2023;185:107378. Disponible en: https://doi.org/10.1016/j.lungcan.2023.107378.
- Garassino MC, Sands J, Paz-Ares L, Lisberg A, Johnson M, Pérol M, et al. PL02.11 Normalized Membrane Ratio of TROP2 by Quantitative Continuous Scoring is Predictive of Clinical Outcomes in TROPION-Lung 01. Journal of Thoracic Oncology. [Internet] 2024;19(10):S2–3. Disponible en: https://doi.org/10.1016/j.jtho.2024.09.015
- Parra-Medina R, Guerron-Gomez G, Mendivelso-González D, Gil-Gómez JH, Alzate JP, Gomez-Suarez M, et al. Deep learning in histopathology images for prediction of oncogenic driver molecular alterations in lung cancer: a systematic review and meta-analysis. Transl Lung Cancer Res. [Internet] 2025;14(5):1756–69. Disponible en: https://doi.org/10.21037/tlcr-2024-1196.
- Parra-Medina R, Sua LF, Polo-Nieto JF, Baldión AM, Moreno MC, Serna A, et al. Consenso de expertos multidisciplinario para la calidad diagnóstica y uso de biomarcadores en cáncer de pulmón. Asociación Colombiana de Patología (ASOCOLPAT). 2023. Revista Colombiana de Neumología. [Internet] 2024;36(2):88–102. Disponible en: https://doi.org/10.30789/rcneumologia.v36.n2.2024.973
- Heredia D, Mas L, Cardona AF, Oyervides V, Motta Guerrero R, Galvez-Nino M, et al. A high number of co-occurring genomic alterations detected by NGS is associated with worse clinical outcomes in advanced EGFR-mutant lung adenocarcinoma: Data from LATAM population. Lung Cancer. [Internet] 2022;174:133–40. Disponible en: https://doi.org/10.1016/j.lungcan.2022.11.002
- Ordóñez-Rubiano EG, Siabato C, Rincón-Arias N, Pulido PA, Pimienta-Redondo HD, Espinosa-Gaona S, et al. Diffuse gliomas: insights into clinical and histopathological features and survival rates from two centers in a middle-income country. Front Oncol. [Internet] 2025;15. Disponible en: https://doi.org/10.3389/fonc.2025.1529456
- Rubiano EGO, Baldoncini M, Cómbita AL, Payán-Gómez C, Gómez-Amarillo DF, Hakim F, et al. Understanding the molecular profiling of diffuse gliomas classification: A brief overview. Surg Neurol Int. [Internet] 2023;14:225. Disponible en: https://doi.org/10.25259/SNI_209_2023.
- Mellinghoff IK, van den Bent MJ, Blumenthal DT, Touat M, Peters KB, Clarke J, et al. Vorasidenib in IDH1- or IDH2-Mutant Low-Grade Glioma. New England Journal of Medicine. [Internet] 2023;389(7):589–601. Disponible en: https://doi.org/10.1056/NEJMoa2304194.
- Akinleye A, Rasool Z. Immune checkpoint inhibitors of PD-L1 as cancer therapeutics. J Hematol Oncol. [Internet] 2019;12(1):92. Disponible en: https://doi.org/10.1186/s13045-019-0779-5.
- Yi M, Zheng X, Niu M, Zhu S, Ge H, Wu K. Combination strategies with PD-1/PD-L1 blockade: current advances and future directions. Mol Cancer. [Internet] 2022;21(1):28. Disponible en: https://doi.org/10.1186/s12943-021-01489-2.
- Akhtar M, Rashid S, Al-Bozom IA. PD-L1 immunostaining: what pathologists need to know. Diagn Pathol. [Internet] 2021;16(1):94. Disponible en: https://doi.org/10.1186/s13000-021-01151-x.
- Marcus L, Lemery SJ, Keegan P, Pazdur R. FDA Approval Summary: Pembrolizumab for the Treatment of Microsatellite Instability-High Solid Tumors. Clinical Cancer Research. [Internet] 2019;25(13):3753–8. Disponible en: https://doi.org/10.1158/1078-0432.CCR-18-4070.
- Hechtman JF. NTRK insights: best practices for pathologists. Mod Pathol. [Internet] 2022;35(3):298–305. Disponible en: https://doi.org/10.1038/s41379-021-00913-8.
- Nguyen MA, Colebatch AJ, Van Beek D, Tierney G, Gupta R, Cooper WA. NTRK fusions in solid tumours: what every pathologist needs to know. Pathology. [Internet] 2023;55(5):596–609. Disponible en: https://doi.org/10.1016/j.pathol.2023.05.002.
- Hewitt R, Watson P. Defining biobank. Biopreserv Biobank. [Internet] 2013;11(5):309–15. Disponible en: https://doi.org/10.1089/bio.2013.0042.
- Kaye J, Organisation for Economic Co-operation and Development. Building a foundation for biobanking: the 2009 OECD guidelines on human biobanks and genetic research databases (HBGRDs). Eur J Health Law. [Internet] 2010;17(2):187–90. Disponible en: https://doi.org/10.1163/157180910x12665776638821.
- Snapes E, Simeon-Dubach D. ISBER Best Practices for Repositories, Moving Toward the Fifth Edition. Biopreserv Biobank. [Internet] 2022 Feb;20(1):107–8. Disponible en: https://doi.org/10.1089/bio.2022.29102.ejs
- Müller H, Dagher G, Loibner M, Stumptner C, Kungl P, Zatloukal K. Biobanks for life sciences and personalized medicine: importance of standardization, biosafety, biosecurity, and data management. Curr Opin Biotechnol. [Internet] 2020;65:45–51. Disponible en: https://doi.org/10.1016/j.copbio.2019.12.004.
- Aarden E. Infrastructuring European scientific integration: Heterogeneous meanings of the European biobanking infrastructure BBMRI-ERIC. Soc Stud Sci. [Internet] 2023;53(4):572–98. Disponible en: https://doi.org/10.1177/03063127231162629.
- Conroy MC, Lacey B, Bešević J, Omiyale W, Feng Q, Effingham M, et al. UK Biobank: a globally important resource for cancer research. Br J Cancer. [Internet] 2023;128(4):519–27. Disponible en: https://doi.org/10.1038/s41416-022-02053-5.
- Perdomo S, Abedi-Ardekani B, de Carvalho AC, Ferreiro-Iglesias A, Gaborieau V, Cattiaux T, et al. The Mutographs biorepository: A unique genomic resource to study cancer around the world. Cell genomics. [Internet] 2024;4(3):100500. Disponible en: https://doi.org/10.1016/j.xgen.2024.100500.
- Yang H, Cheng J, Zhuang H, Xu H, Wang Y, Zhang T, et al. Pharmacogenomic profiling of intra-tumor heterogeneity using a large organoid biobank of liver cancer. Cancer Cell. [Internet] 2024;42(4):535-551.e8. Disponible en: https://doi.org/10.1016/j.ccell.2024.03.004.
- Policiuc L, Nutu A, Zanoaga O, Mehterov N, Braicu C, Berindan-Neagoe I. Current aspects in biobanking for personalized oncology investigations and treatments. Med Pharm Rep. [Internet] 2023;96(3):235–45. Disponible en: https://doi.org/10.15386/mpr-2647.
- Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. [Internet] 2000;406(6797):747–52. Disponible en: https://doi.org/10.1038/35021093.
- Dienstmann R, Vermeulen L, Guinney J, Kopetz S, Tejpar S, Tabernero J. Consensus molecular subtypes and the evolution of precision medicine in colorectal cancer. Nat Rev Cancer. [Internet] 2017;17(2):79–92. Disponible en: https://doi.org/10.1038/nrc.2016.126
- Knoppers BM, Harris JR, Budin-Ljøsne I, Dove ES. A human rights approach to an international code of conduct for genomic and clinical data sharing. Hum Genet. [Internet] 2014;133(7):895–903. Disponible en: https://doi.org/10.1007/s00439-014-1432-6
- Brancato V, Esposito G, Coppola L, Cavaliere C, Mirabelli P, Scapicchio C, et al. Standardizing digital biobanks: integrating imaging, genomic, and clinical data for precision medicine. J Transl Med. [Internet] 2024;22(1):136. Disponible en: https://doi.org/10.1186/s12967-024-04891-8.
- Manotas MC, Rivera AL, Gómez AM, Abisambra P, Guevara G, Medina V, et al. SDHB exon 1 deletion: A recurrent germline mutation in Colombian patients with pheochromocytomas and paragangliomas. Front Genet. [Internet] 2022;13:999329. Disponible en: https://doi.org/10.3389/fgene.2022.999329.
- Ambriz-Barrera F, Rojas-Jiménez E, Díaz-Velásquez CE, De-La-Cruz-Montoya AH, Martínez-Gregorio H, Ruiz-De-La-Cruz M, et al. Mutational spectrum of breast cancer by shallow whole-genome sequencing of cfDNA and tumor gene panel analysis. PLoS One. [Internet] 2024;19(9):e0308176. Disponible en: https://doi.org/10.1371/journal.pone.0308176
- Ardila HJ, Sanabria-Salas MC, Meneses X, Rios R, Huertas-Salgado A, Serrano ML. Circulating miR-141-3p, miR-143-3p and miR-200c-3p are differentially expressed in colorectal cancer and advanced adenomas. Mol Clin Oncol. [Internet] 2019 Aug;11(2):201–7. Disponible en: https://doi.org/10.3892/mco.2019.1876
- Díaz-Gay M, dos Santos W, Moody S, Kazachkova M, Abbasi A, Steele CD, et al. Geographic and age variations in mutational processes in colorectal cancer. Nature. [Internet] 2025;643(8070):230–40. Disponible en: https://doi.org/10.1038/s41586-025-09025-8.
- Guerra J, Estrada-Florez AP, Lott PC, Pinto C, Pinheiro M, Chiu KA, et al. The role of multi-organ cancer predisposition genes in the risk of inherited and histologically diverse gastric cancer. EBioMedicine. [Internet] 2025;116:105759. Disponible en: https://doi.org/10.1016/j.ebiom.2025.105759.
- Yuille M, van Ommen GJ, Bréchot C, Cambon-Thomsen A, Dagher G, Landegren U, et al. Biobanking for Europe. Brief Bioinform. [Internet] 2008;9(1):14–24. Disponible en: https://doi.org/10.1093/bib/bbm050
- Hewitt R, Watson P. Defining biobank. Biopreserv Biobank. [Internet] 2013;11(5):309–15. Disponible en: https://doi.org/10.1089/bio.2013.0042
- Skloot R. The Immortal Life of Henrietta Lacks. New York: Crown; 2010. 381p.
- Sterling RL. Genetic research among the Havasupai--a cautionary tale. Virtual Mentor. [Internet] 2011;13(2):113–7. Disponible en: https://doi.org/10.1001/virtualmentor.2011.13.2.hlaw1-1102.
- Garrison NA, Hudson M, Ballantyne LL, Garba I, Martinez A, Taualii M, et al. Genomic Research Through an Indigenous Lens: Understanding the Expectations. Annu Rev Genomics Hum Genet. [Internet] 2019;20:495–517. Disponible en: https://doi.org/10.1146/annurev-genom-083118-015434.
- Congreso de la República de Colombia. Ley 2287 de 2023. Por medio de la cual se crea el Sistema Nacional de Biobancos y se regula el funcionamiento de los biobancos con fines de investigación biomédica, biotecnológica y epidemiológica y se dictan otras disposiciones. Diario Oficial No. 52.354 del 18 de enero de 2023. Disponible en: https://www.redjurista.com/Documents/ley_2287_de_2023_.aspx#/
- World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. [Internet] 2013;310(20):2191–4. Disponible en: https://doi.org/10.1001/jama.2013.281053.
- Sterling RL. Genetic research among the Havasupai--a cautionary tale. Virtual Mentor. [Internet] 2011;13(2):113–7. Disponible en: https://doi.org/10.1001/virtualmentor.2011.13.2.hlaw1-1102.
- Mikkelsen RB, Gjerris M, Waldemar G, Sandøe P. Broad consent for biobanks is best - provided it is also deep. BMC Med Ethics. [Internet] 2019;20(1):71. Disponible en: https://doi.org/10.1186/s12910-019-0414-6.
- Olivieri DJ, Berridge-Green A, Othus M, Radich JP, Advani AS, Erba HP, et al. Biobanking and consent to future biospecimen use among adults enrolled in SWOG trials from 2000 to 2024. Blood Cancer J. [Internet] 2025;15(1):85. Disponible en: https://doi.org/10.1038/s41408-025-01294-w.
- European Parliament and Council. Regulation (EU) 2016/679 (General Data Protection Regulation). Official Journal of the European Union. 2016 Apr 27.
- U.S. Department of Health and Human Services. Health Insurance Portability and Accountability Act (HIPAA) Privacy Rule. Federal Register. 2000;65(250):82462–829.
- Robertson HT, de los Campos G, Allison DB. Turning the analysis of obesity-mortality associations upside down: modeling years of life lost through conditional distributions. Obesity (Silver Spring). [Internet] 2013;21(2):398–404. Disponible en: https://doi.org/10.1002/oby.20019.
- Harris JR, Burton P, Knoppers BM, Lindpaintner K, Bledsoe M, Brookes AJ, et al. Toward a roadmap in global biobanking for health. Eur J Hum Genet. [Internet] 2012;20(11):1105–11. Disponible en: https://doi.org/10.1038/ejhg.2012.96.
- de Vries J, Munung SN, Matimba A, McCurdy S, Ouwe Missi Oukem-Boyer O, Staunton C, et al. Regulation of genomic and biobanking research in Africa: a content analysis of ethics guidelines, policies and procedures from 22 African countries. BMC Med Ethics. [Internet] 2017;18(1):8. Disponible en: https://doi.org/10.1186/s12910-016-0165-6.
- Council for International Organizations of Medical Sciences (CIOMS). International Ethical Guidelines for Health-related Research Involving Humans. Geneva: CIOMS. [Internet] 2016. Disponible en: https://doi.org/10.56759/rgxl7405
- Ramsay M. African genomic data sharing and the struggle for equitable benefit. Patterns (N Y). [Internet] 2022;3(1):100412. Disponible en: https://doi.org/10.1016/j.patter.2021.100412.
- Carroll SR, Garba I, Figueroa-Rodríguez OL, Holbrook J, Lovett R, Materechera S, et al. The CARE Principles for Indigenous Data Governance. Data Sci J. [Internet] 2020;19. Disponible en: https://doi.org/10.5334/dsj-2020-043
- Sirugo G, Williams SM, Tishkoff SA. The Missing Diversity in Human Genetic Studies. Cell. [Internet] 2019;177(1):26–31. Disponible en: https://doi.org/10.1016/j.cell.2019.04.032.
- Hudson M, Garrison NA, Sterling R, Caron NR, Fox K, Yracheta J, et al. Rights, interests and expectations: Indigenous perspectives on unrestricted access to genomic data. Nat Rev Genet. [Internet] 2020;21(6):377–84. Disponible en: https://doi.org/10.1038/s41576-020-0228-x.
- Arbour L, Cook D. DNA on loan: issues to consider when carrying out genetic research with aboriginal families and communities. Community Genet. [Internet] 2006;9(3):153–60. Disponible en: https://doi.org/10.1159/000092651.
- Popejoy AB, Fullerton SM. Genomics is failing on diversity. Nature. [Internet] 2016;538(7624):161–4. Disponible en: https://doi.org/10.1038/538161a.
- Wolf SM, Crock BN, Van Ness B, Lawrenz F, Kahn JP, Beskow LM, et al. Managing incidental findings and research results in genomic research involving biobanks and archived data sets. Genet Med. [Internet] 2012;14(4):361–84. Disponible en: https://doi.org/10.1038/gim.2012.23.
- Manchanda R, Burnell M, Gaba F, Desai R, Wardle J, Gessler S, et al. Randomised trial of population-based BRCA testing in Ashkenazi Jews: long-term outcomes. BJOG. [Internet] 2020;127(3):364–75. Disponible en: https://doi.org/10.1111/1471-0528.15905.

















