Multiple myeloma: from the genome to precision medicine
Mieloma múltiple: del genoma a la medicina de precisión
How to Cite
Download Citation

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Show authors biography
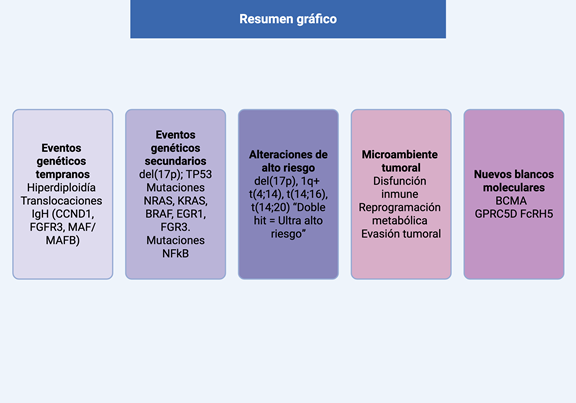
Introduction: multiple myeloma (MM) is a clonal plasma cell proliferative disorder characterized by marked biological and clinical heterogeneity. Genomic instability associated with plasma cell maturation leads to recurrent genetic alterations that play a central role in MM pathogenesis. The aim of this review is to describe the genetic architecture of MM and its impact on prognosis and treatment.
Methods: a literature review was conducted focusing on recent advances in the molecular basis of MM, integrating findings from genomics, epigenetics, immunology, and the bone marrow microenvironment, with emphasis on clinical applicability.
Results: genetic alterations in MM are classified as primary and secondary events, which are responsible for disease initiation and progression and may occur in the presence or absence of hyperdiploidy. Interactions with the bone marrow microenvironment promote tumor progression and immune evasion. Detailed molecular characterization has enabled the development of prognostic scoring systems that identify high-risk patients who may benefit from more intensive therapeutic strategies. In addition, novel therapeutic targets have been identified, leading to the development of T-cell–redirecting therapies, including CAR T-cell therapies and bispecific antibodies, which have demonstrated deep and durable responses. A subset of patients with favorable cytogenetics and sustained responses achieves survival beyond 10 years, supporting the concept of functional cure.
Conclusion: integration of multi-omic data and updated prognostic models will enable further advances toward precision medicine, increasing the proportion of patients with long-term remissions while reducing the toxicity associated with continuous treatment.
Article visits 0 | PDF visits 0
Downloads
- Ludwig H, Novis Durie S, Meckl A, Hinke A, Durie B. Multiple myeloma incidence and mortality around the globe: interrelations between health access and quality, economic resources, and patient empowerment. Oncologist [Internet]. 2020;25(9):e1406–e1413. Disponible en: https://doi.org/10.1634/theoncologist.2020-0141
- Siegel RL, Kratzer TB, Giaquinto AN, Sung H, Jemal A. Cancer statistics, 2025. CA Cancer J Clin [Internet]. 2025;75(1):10–45. Disponible en: https://doi.org/10.3322/caac.21871
- International Agency for Research on Cancer. GLOBOCAN 2022: Colombia fact sheet. Global Cancer Observatory [Internet]. 2022. Disponible en: https://gco.iarc.who.int.
- Cuenta de Alto Costo. Informe especial: neoplasias hematológicas en el marco del aseguramiento en Colombia. [Internet]. 2025. Disponible en: https://cuentadealtocosto.org
- Palumbo A, Anderson K. Multiple myeloma. N Engl J Med [Internet]. 2011;364(11):1046–1060. Disponible en: https://doi.org/10.1056/NEJMra1011442
- Surveillance, Epidemiology, and End Results Program. Cancer stat facts: myeloma. [Internet]. Disponible en: https://seer.cancer.gov/statfacts/html/mulmy.html
- Avet-Loiseau H, Attal M, Campion L, et al. Long-term analysis of the IFM 99 trials for myeloma: cytogenetic abnormalities play a major role in defining long-term survival. J Clin Oncol [Internet]. 2012;30(16):1949–1952. Disponible en: https://doi.org/10.1200/JCO.2011.36.5726
- Pasvolsky O, Wang Z, Milton DR, et al. Multiple myeloma patients with a long remission after autologous hematopoietic stem cell transplantation. Blood Cancer J [Internet]. 2024;14(1):82. Disponible en: https://doi.org/10.1038/s41408-024-01062-2
- Kasomva K, Yadav K, Janz S, Dhakal B, Rao S. Molecular and immunological determinants of long-term survival in multiple myeloma. Blood Adv [Internet]. 2025. Disponible en: https://doi.org/10.1182/bloodadvances.2025016829.
- Gong L, Qiu L, Hao M. Novel insights into the initiation, evolution, and progression of multiple myeloma by multi-omics investigation. Cancers (Basel) [Internet]. 2024;16(3):498. Disponible en: https://doi.org/10.3390/cancers16030498
- Heider M, Nickel K, Högner M, Bassermann F. Multiple myeloma: molecular pathogenesis and disease evolution. Oncol Res Treat [Internet]. 2021;44(12):672–681. Disponible en: https://doi.org/10.1159/000520312
- Xu A, Guo T, Zhang S, et al. Prevalence of monoclonal gammopathy of undetermined significance in Shenzhen, China. Hematology [Internet]. 2024;29(1):2352686. Disponible en: https://doi.org/10.1080/16078454.2024.2352686
- Landgren O, Kyle RA, Pfeiffer RM, et al. Monoclonal gammopathy of undetermined significance consistently precedes multiple myeloma: a prospective study. Blood [Internet]. 2009;113(22):5412–5417. Disponible en: https://doi.org/10.1182/blood-2008-12-194241
- van Nieuwenhuijzen N, Spaan I, Raymakers R, Peperzak V. From MGUS to multiple myeloma, a paradigm for clonal evolution of premalignant cells. Cancer Res [Internet]. 2018;78(10):2449–2456. Disponible en: https://doi.org/10.1158/0008-5472.can-17-3115
- Fonseca R, Debes-Marun CS, Picken EB, et al. The recurrent IgH translocations are highly associated with nonhyperdiploid variant multiple myeloma. Blood [Internet]. 2003;102(7):2562–2567. Disponible en: https://doi.org/10.1182/blood-2003-02-0493
- Prideaux SM, Conway O’Brien E, Chevassut TJ. The genetic architecture of multiple myeloma. Adv Hematol [Internet]. 2014;2014:864058. Disponible en: https://doi.org/10.1155/2014/864058
- Smadja NV, Fruchart C, Isnard F, et al. Chromosomal analysis in multiple myeloma: cytogenetic evidence of two different diseases. Leukemia [Internet]. 1998;12(6):960–969. Disponible en: https://doi.org/10.1038/sj.leu.2401041
- Testa U, Pelosi E, Castelli G, Leone G. Recent advances in the definition of the molecular alterations occurring in multiple myeloma. Mediterr J Hematol Infect Dis [Internet]. 2024;16(1):e2024062. Disponible en: https://doi.org/10.4084/MJHID.2024.062
- Ntanasis-Stathopoulos I, Terpos E, Malandrakis P, et al. Prognostic impact of t(11;14) in newly diagnosed patients with multiple myeloma in the era of modern treatments. Blood [Internet]. 2024;144:6968. Disponible en: https://doi.org/10.1182/blood-2024-202627
- Hebraud B, Magrangeas F, Cleynen A, et al. Role of additional chromosomal changes in the prognostic value of t(4;14) and del(17p) in multiple myeloma. Blood [Internet]. 2015;125(13):2095–2100. Disponible en: https://doi.org/10.1182/blood-2014-07-587964
- De Novellis D, Scala P, Giudice V, Selleri C. High-risk genetic multiple myeloma: from molecular classification to innovative treatment with monoclonal antibodies and T-cell redirecting therapies. Cells [Internet]. 2025;14(11). Disponible en: https://doi.org/10.3390/cells14110776
- Chapman MA, Lawrence MS, Keats JJ, et al. Initial genome sequencing and analysis of multiple myeloma. Nature [Internet]. 2011;471(7339):467–472. Disponible en: https://doi.org/10.1038/nature09837
- Shah V, Sherborne AL, Walker BA, et al. Prediction of outcome in newly diagnosed myeloma: a meta-analysis of the molecular profiles of 1905 trial patients. Leukemia [Internet]. 2018;32(1):102–110. Disponible en: https://doi.org/10.1038/leu.2017.179
- Walker BA, Mavrommatis K, Wardell CP, et al. A high-risk, double-hit, group of newly diagnosed myeloma identified by genomic analysis. Leukemia [Internet]. 2019;33(1):159–170. Disponible en: https://doi.org/10.1038/s41375-018-0196-8
- D’Agostino M, Cairns DA, Lahuerta JJ, et al. Second revision of the International Staging System (R2-ISS) for overall survival in multiple myeloma: A European Myeloma Network (EMN) report within the HARMONY Project. J Clin Oncol [Internet]. 2022;40(29):3406–3418. Disponible en: https://doi.org/10.1200/jco.21.02614
- Avet-Loiseau H, Davies FE, Samur MK, et al. International Myeloma Society/International Myeloma Working Group Consensus recommendations on the definition of high-risk multiple myeloma. J Clin Oncol [Internet]. 2025;43(24):2739–2751. Disponible en: https://doi.org/10.1200/jco-24-01893
- Terebelo HR, Omel J, Wagner LI, et al. Characteristics and Treatment Patterns of Long-surviving Patients With Multiple Myeloma: Over 13 Years of Follow-up in the ConnectⓇ MM Registry. Clin Lymphoma Myeloma Leuk [Internet]. 2025;25(1):58–66. Disponible en: https://doi.org/10.1016/j.clml.2024.11.001
- Vu T, Gonsalves W, Kumar S, et al. Characteristics of exceptional responders to lenalidomide-based therapy in multiple myeloma. Blood Cancer J [Internet]. 2015;5(10):e363. Disponible en: https://doi.org/10.1038/bcj.2015.91
- Paner A, Patel P, Dhakal B. The evolving role of translocation t(11;14) in multiple myeloma. Blood Rev [Internet]. 2020;41:100643. Disponible en: https://doi.org/10.1016/j.blre.2019.100643
- Atamaniuk J, Gleiss A, Porpaczy E, et al. Overexpression of G protein-coupled receptor 5D in the bone marrow is associated with poor prognosis in multiple myeloma. Eur J Clin Invest [Internet]. 2012;42(9):953–960. Disponible en: https://doi.org/10.1111/j.1365-2362.2012.02679.x
- Rodriguez-Otero P, van de Donk NWCJ, Pillarisetti K, et al. Correction: GPRC5D as a novel target for the treatment of multiple myeloma. Blood Cancer J [Internet]. 2024;14(1):40. Disponible en: https://doi.org/10.1038/s41408-024-01018-6
- Rodriguez-Otero P, van de Donk NWCJ, Pillarisetti K, et al. GPRC5D as a novel target for the treatment of multiple myeloma. Blood Cancer J [Internet]. 2024;14(1):24. Disponible en: https://doi.org/10.1038/s41408-024-01018-6
- Frerichs KA, Broekmans MEC, Marin Soto JA, et al. Preclinical activity of JNJ-7957, a novel BCMA×CD3 bispecific antibody. Clin Cancer Res [Internet]. 2020;26(9):2203–2215. Disponible en: https://doi.org/10.1158/1078-0432.ccr-19-2299
- Lonial S, Lee HC, Badros A, et al. Belantamab mafodotin for relapsed or refractory multiple myeloma (DREAMM-2): a two-arm, randomised, open-label, phase 2 study. Lancet Oncol [Internet]. 2020;21(2):207–221. Disponible en: https://doi.org/10.1016/S1470-2045(19)30788-0
- Munshi NC, Anderson LD, Shah N, et al. Idecabtagene vicleucel in relapsed and refractory multiple myeloma. N Engl J Med [Internet]. 2021;384(8):705–716. Disponible en: https://doi.org/10.1056/NEJMoa2024850
- Berdeja JG, Madduri D, Usmani SZ, et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. Lancet [Internet]. 2021;398(10297):314–324. Disponible en: https://doi.org/10.1016/S0140-6736(21)00933-8
- Waldschmidt JM, Rasche L, Kortüm KM, Einsele H. Comprehensive review of bispecific antibody constructs in multiple myeloma. Clin Lymphoma Myeloma Leuk [Internet]. 2025;25(5):309–315. Disponible en: https://doi.org/10.1016/j.clml.2024.11.012
- Kumar SK, Rajkumar V, Kyle RA, et al. Multiple myeloma. Nat Rev Dis Primers [Internet]. 2017;3:17046. Disponible en: https://doi.org/10.1038/nrdp.2017.46
- Chesi M, Bergsagel PL. Molecular pathogenesis of multiple myeloma: basic and clinical updates. Int J Hematol [Internet]. 2013;97(3):313–323. Disponible en: https://doi.org/10.1007/s12185-013-1291-2
- Moreau P, Cavo M, Sonneveld P, et al. Combination of International Scoring System 3, High Lactate Dehydrogenase, and t(4;14) and/or del(17p) Identifies Patients With Multiple Myeloma (MM) Treated With Front-Line Autologous Stem-Cell Transplantation at High Risk of Early MM Progression–Related Death. J Clin Oncol [Internet]. 2014;32(20):2173–2180. Disponible en: https://doi.org/10.1200/jco.2013.53.0329
- Palumbo A, Avet-Loiseau H, Oliva S, et al. Revised International Staging System for multiple myeloma: A Report From International Myeloma Working Group. J Clin Oncol [Internet]. 2015;33(26):2863–2869. Disponible en: https://doi.org/10.1200/JCO.2015.61.2267
- Zanwar S, Rajkumar SV. Current risk stratification and staging of multiple myeloma and related plasma cell disorders. Leukemia [Internet]. 2025. Disponible en: https://doi.org/10.1038/s41375-025-02654-y

















