Molecular biology of melanoma
Biología molecular del melanoma
How to Cite
Download Citation

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Show authors biography
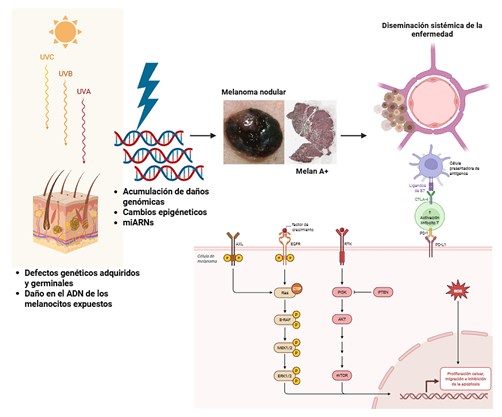
Introduction: Melanoma is an aggressive neoplasm with an increasing incidence in Colombia. Recent data reveal relevant differences in the distribution of its subtypes compared with international populations, with a higher frequency of acral lentiginous melanoma and lentigo maligna, suggesting the influence of genetic, environmental, or demographic factors specific to the Colombian population.
Objective: To describe the molecular alterations related to the pathogenesis of cutaneous melanoma, the current molecular classification system, including relevant co-mutations, as well as the pathophysiological mechanisms that support the use of therapeutic alternatives currently employed in cutaneous melanoma.
Methods: A literature review was conducted using databases such as PubMed, Embase, Scopus, Web of Science, and Google Scholar. Articles addressing the pathophysiological mechanisms underlying current molecular classification systems for melanoma, the most frequently reported mutations in human cutaneous melanoma, and predictors of response to targeted therapies were included.
Results: A total of 29 articles were selected describing the main molecular alterations in cutaneous melanoma, including mutations in genes involved in genomic regulation and metabolic response. A narrative synthesis of the alterations supporting oncogenesis, tumor progression, and mechanisms of therapeutic resistance is presented.
Conclusions: Cutaneous melanoma in Colombia exhibits clinical and epidemiological features that distinguish it from global patterns, with a predominance of the acral lentiginous subtype. While histological classification provides an initial approach to disease heterogeneity, the diversity of canonical and non-canonical mutations, as well as their impact on clinical behavior and therapeutic response, allows for a more accurate characterization of this complexity.
Article visits 0 | PDF visits 0
Downloads
- Rashid S, Shaughnessy M, Tsao H. Melanoma classification and management in the era of molecular medicine. Dermatol Clin. [internet] 2023;41(1):49–63. Disponible en: https://doi.org/10.1016/j.det.2022.07.017
- Uribe Ortiz PA, Nova Villanueva JA, Colmenares Mejia CC, Palma Escobar L, Gil Quiñones SR. Características del melanoma cutáneo en dos instituciones de Bogotá, Colombia: análisis 2012–2016. Rev Colomb Cancerol. [internet] 2021;25(4):188–195. Disponible en: https://doi.org/10.35509/01239015.692
- Ospina Serrano AV, Contreras F, Triana I, Sánchez-Vanegas GS, Ortiz JD, Ramos P, et al. Clinical outcomes and prognostic factors of patients with early malignant melanoma in one Latin American country: results of the Epidemiological Registry of Malignant Melanoma in Colombia Study. JCO Glob Oncol. [internet] 2023;9:e2200377. Disponible en: https://doi.org/10.1200/GO.22.00377
- Mirek J, Bal W, Olbryt M. Melanoma genomics – will we go beyond BRAF in clinics? J Cancer Res Clin Oncol. [internet] 2024;150:433. Disponible en: https://doi.org/10.1007/s00432-024-05957-2
- The Cancer Genome Atlas Network. Genomic classification of cutaneous melanoma. Cell. [internet] 2015;161(7):1681–1696. Disponible en: https://doi.org/10.1016/j.cell.2015.05.044
- Phadke MS, Smalley KSM. Targeting NRAS mutations in advanced melanoma. J Clin Oncol. [internet] 2023;41(14:2661-2664. Disponible en: https://doi.org/10.1200/JCO.23.00205
- Li C, Kuai L, Cui R, Miao X. Melanogenesis and the targeted therapy of melanoma. Biomolecules. [internet] 2022;12(12):1874. Disponible en: https://doi.org/10.3390/biom12121874
- Castellani G, Buccarelli M, Arasi MB, Rossi S, Pisanu ME, Bellenghi M, et al. BRAF mutations in melanoma: biological aspects, therapeutic implications, and circulating biomarkers. Cancers (Basel). [internet] 2023;15(16):4026. Disponible en: https://doi.org/10.3390/cancers15164026
- Menzer C, Menzies AM, Carlino MS, Reijers I, Groen EJ, Eigentler T, et al. Targeted therapy in advanced melanoma with rare BRAF mutations. J Clin Oncol. [internet] 2019;37(33):3142-3151. Disponible en: https://doi.org/10.1200/jco.19.00489
- Gouda MA, Subbiah V. Precision oncology for BRAF-mutant cancers with BRAF and MEK inhibitors: from melanoma to tissue-agnostic therapy. ESMO Open. [internet] 2023;8(2):100788. Disponible en: https://doi.org/10.1016/j.esmoop.2023.100788
- Luebker SA, Koepsell SA. Diverse mechanisms of BRAF inhibitor resistance in melanoma identified in clinical and preclinical studies. Front Oncol. [internet] 2019;9:268. Disponible en: https://doi.org/10.3389/fonc.2019.00268
- Al Mahi A, Ablain J. RAS pathway regulation in melanoma. Dis Model Mech. [internet] 2022;15(2):dmm049229. Disponible en: https://doi.org/10.1242/dmm.049229
- Báez-Flores J, Rodríguez-Martín M, Lacal J. The therapeutic potential of neurofibromin signaling pathways and binding partners. Commun Biol. [internet] 2023;6(1):436. Disponible en: https://doi.org/10.1038/s42003-023-04815-0
- Ferrara G, Argenziano G. The WHO 2018 classification of cutaneous melanocytic neoplasms: suggestions from routine practice. Front Oncol. [internet] 2021;11:675296. Disponible en: https://doi.org/10.3389/fonc.2021.675296
- Wang L, Kim KB, Kashani-Sabet M, Dighe PR, Aboosaiedi A. Outcomes of patients with advanced NF1-mutant melanoma treated with MEK inhibitors. [Abstract]w Target Oncol. [internet] 2023;41(16suppl):e21513. Disponible en: https://doi.org/10.1200/jco.2023.41.16_suppl.e21513
- Huang AC, Zappasodi R. A decade of checkpoint blockade immunotherapy in melanoma: understanding the molecular basis for immune sensitivity and resistance. Nat Immunol. [internet] 2022;23(5):660–670. Disponible en: https://doi.org/10.1038/s41590-022-01141-1
- Olbryt M, Rajczykowski M, Widłak W. Biological factors behind melanoma response to immune checkpoint inhibitors. Int J Mol Sci. [internet] 2020;21(11):4071. Disponible en: https://doi.org/10.3390/ijms21114071
- Gandara DR, Agarwal N, Gupta S, Klempner SJ, Andrews MC, Mahipal A, et al. Tumor mutational burden and survival on immune checkpoint inhibition in >8000 patients across 24 cancer types. J Immunother Cancer. [internet] 2025;13(2):e010311. Disponible en: https://doi.org/10.1136/jitc-2024-010311corr1
- Andrews MC, Li G, Graf RP, Fisher VA, Mitchell J, Aboosaiedi A, et al. Predictive impact of tumor mutational burden on real-world outcomes of first-line immune checkpoint inhibition in metastatic melanoma. JCO Precis Oncol. [internet] 2024;8:e2300640. Disponible en: https://doi.org/10.1200/po.23.00640
- Zielińska MK, Ciążyńska M, Sulejczak D, Rutkowski P, Czarnecka AM. Mechanisms of resistance to anti-PD-1 immunotherapy in melanoma and strategies to overcome it. Biomolecules. [internet] 2025;15(2):269. Disponible en: https://doi.org/10.3390/biom15020269
- Zhao X, Ma Y, Luo J, Xu K, Tian P, Lu C, et al. Blocking the WNT/β-catenin pathway in cancer treatment: pharmacological targets and drug therapeutic potential. Heliyon. [internet] 2024;10(16),e35989. Disponible en: https://doi.org/10.1016/j.heliyon.2024.e35989
- Reinfeld BI, Rathmell WK, Kim TK, Rathmell JC. The therapeutic implications of immunosuppressive tumor aerobic glycolysis. Cell Mol Immunol. [internet] 2022;19(1):46–58. Disponible en: https://doi.org/10.1038/s41423-021-00727-3
- Liu ZL, Chen HH, Zheng LL, Sun LP, Shi L. Angiogenic signaling pathways and anti-angiogenic therapy for cancer. Signal Transduct Target Ther. [internet] 2023;8(1):198. Disponible en: https://doi.org/10.1038/s41392-023-01460-1
- Baradaran A, Asadzadeh Z, Hemmat N, Baghbanzadeh A, Shadbad MA, Khosravi N, et al. The cross-talk between tumor-associated macrophages and tumor endothelium: recent advances in macrophage-based cancer immunotherapy. Biomed Pharmacother. [internet] 2022;146:112588. Disponible en: https://doi.org/10.1016/j.biopha.2021.112588
- Wu Z, Bian Y, Chu T, Wang Y, Man S, Song Y, et al. The role of angiogenesis in melanoma: clinical treatments and future expectations. Front Pharmacol. [internet] 2022;13:1028647. Disponible en: https://doi.org/10.3389/fphar.2022.1028647
- Wang Z, Li J, Guo J, Wei P. Direct antitumor activity of bevacizumab: an overlooked mechanism? Front Pharmacol. [internet] 2024;15:1394878. Disponible en: https://doi.org/10.3389/fphar.2024.1394878
- Han X, Ge P, Liu S, Yang D, Zhang J, Wang X, et al. Efficacy and safety of bevacizumab in patients with malignant melanoma: a systematic review and PRISMA-compliant meta-analysis of randomized controlled trials and non-comparative clinical studies. Front Pharmacol. [internet] 2023;14:1163805. Disponible en: https://doi.org/10.3389/fphar.2023.1163805
- Capozzi M, De Divitiis C, Ottaiano A. Lenvatinib, a molecule with versatile application: from preclinical evidence to future development in anti-cancer treatment. Cancer Manag Res. [internet] 2019;11:3847–3860. Disponible en: https://doi.org/10.2147/cmar.s188316
- Arance A, De la Cruz-Merino L, Petrella TM, Jamal R, Ny L, Carneiro A, et al. Phase II LEAP-004 study of lenvatinib plus pembrolizumab for melanoma with confirmed progression on PD-1 or PD-L1 inhibitors. J Clin Oncol. [internet] 2022;41(1):75-85. Disponible en: https://doi.org/10.1200/JCO.22.00221

















