Applications of genomics in the design and implementation of cancer clinical trials: towards a new era in clinical research
Aplicaciones de la genómica en el diseño e implementación de ensayos clínicos oncológicos: hacia una nueva era en la investigación clínica
How to Cite
Download Citation

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Show authors biography
Introduction: genomics has transformed clinical cancer research by enabling detailed molecular characterization of tumors. This has driven the development of more precise clinical trial designs to evaluate targeted therapies in specific molecular subgroups, overcoming the limitations of traditional, organ-based approaches.
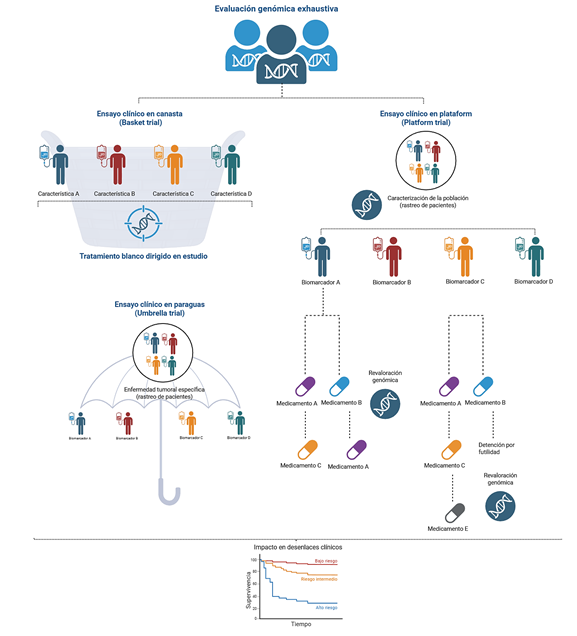
Methods: a narrative review of the scientific literature published between 2008 and 2024 was conducted, focusing on original studies, systematic reviews, and clinical guidelines that address the use of genomics in the design, execution, and interpretation of clinical trials. Emphasis was placed on solid and hematologic malignancies, including basket, umbrella, and adaptive trial designs.
Results: the incorporation of genomic biomarkers has enabled innovative trial designs, such as tumor-agnostic studies, allowing patient selection based on molecular alterations regardless of tumor histology. Trials like NCI-MATCH, SHIVA, TAPUR, and I-SPY2 show that this approach enhances response rates and accelerates drug development. However, challenges remain regarding biomarker validation, genomic data management, access disparities, and clinical interpretation of variants.
Conclusion: the integration of genomics into clinical trials is a cornerstone of precision oncology. Implementing innovative trial designs requires collaborative efforts across technology, bioinformatics, regulatory frameworks, and ethics to ensure effective and equitable application in clinical research.
Article visits 0 | PDF visits 0
Downloads
- Simon R. The Use of Genomics in Clinical Trial Design. Clinical Cancer Research [Internet]. 2008;14:5984–93. Disponible en: https://doi.org/10.1158/1078-0432.ccr-07-4531
- Roychowdhury S. Cancer Genomics Meets Clinical Trials: The Challenge ahead. Personalized Medicine [Internet]. 2012;9:459–61. Disponible en: https://doi.org/10.2217/pme.12.50
- Marabelle A, Fakih M, Lopez J, Shah M, Shapira-Frommer R, Nakagawa K, et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. The Lancet Oncology [Internet]. 2020;21:1353–65. Disponible en: https://doi.org/10.1016/s1470-2045(20)30445-9
- Drilon A, Laetsch TW, Kummar S, DuBois SG, Lassen UN, Demetri GD, et al. Efficacy of Larotrectinib in TRK Fusion–Positive Cancers in Adults and Children. N Engl J Med [Internet]. 2018;378:731–9. Disponible en: https://doi.org/10.1056/nejmoa1714448
- Doebele RC, Drilon A, Paz-Ares L, Siena S, Shaw AT, Farago AF, et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1–2 trials. The Lancet Oncology [Internet]. 2020;21(2):271–82. Disponible en: https://doi.org/10.1016/S1470-2045(19)30691-6
- Farhangfar CJ, Scarola GT, Morris VA, Farhangfar F, Dumas K, Symanowski J, et al. Impact of a Clinical Genomics Program on Trial Accrual for Targeted Treatments: Practical Approach Overcoming Barriers to Accrual for Underserved Patients. JCO Clinical Cancer Informatics [Internet]. 2022:e2200011. Disponible en: https://doi.org/10.1200/cci.22.00011
- Fountzilas E, Tsimberidou AM, Vo HH, Kurzrock R. Clinical trial design in the era of precision medicine. Genome Med [Internet]. 2022;14:101. Disponible en: https://doi.org/10.1186/s13073-022-01102-1
- Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, et al. Intratumor Heterogeneity and Branched Evolution Revealed by Multiregion Sequencing. N Engl J Med [Internet]. 2012;366:883–92. Disponible en: https://doi.org/10.1056/nejmoa1113205
- Fontes Jardim DL, Schwaederle M, Wei C, Lee JJ, Hong DS, Eggermont AM, et al. Impact of a Biomarker-Based Strategy on Oncology Drug Development: A Meta-analysis of Clinical Trials Leading to FDA Approval. JNCIJ [Internet]. 2015;107:djv253. Disponible en: https://doi.org/10.1093/jnci/djv253
- Schwaederle M, Zhao M, Lee JJ, Eggermont AM, Schilsky RL, Mendelsohn J, et al. Impact of Precision Medicine in Diverse Cancers: A Meta-Analysis of Phase II Clinical Trials. JCO [Internet]. 2015;33(32):3817–25. Disponible en: https://doi.org/10.1200/jco.2015.61.5997
- Asad S, Kananen K, Mueller KR, Symmans WF, Wen Y, Perou CM, et al. Challenges and Gaps in Clinical Trial Genomic Data Management. JCO Clinical Cancer Informatics [Internet]. 2022;e2100193. Disponible en: https://doi.org/10.1200/cci.21.00193
- Enoma D. Genomics in Clinical trials for Breast Cancer. Briefings in Functional Genomics [Internet]. 2024;23:325–34. Disponible en: https://doi.org/10.1093/bfgp/elad054
- Simon R, Roychowdhury S. Implementing personalized cancer genomics in clinical trials. Nat Rev Drug Discov [Internet]. 2013;12:358–69. Disponible en: https://doi.org/10.1038/nrd3979
- Bhatt DL, Mehta C. Adaptive Designs for Clinical Trials. Drazen JM, Harrington DP, McMurray JJV, Ware JH, Woodcock J, editores. N Engl J Med [Internet]. 2016;375:65–74. Disponible en: https://doi.org/10.1056/nejmra1510061
- American Society of Clinical Oncology. Targeted Agent and Profiling Utilization Registry (TAPUR) Study [Internet]. clinicaltrials.gov; 2025. Report No.: NCT02693535. Disponible en: https://doi.org/10.1200/adn.19.190373
- Ferrarotto R, Redman MW, Gandara DR, Herbst RS, Papadimitrakopoulou VA. Lung-MAP—framework, overview, and design principles. Chinese Clinical Oncology [Internet]. 2015;4(3):36. Disponible en: https://doi.org/10.3978/j.issn.2304-3865.2015.09.02
- Barker A, Sigman C, Kelloff G, Hylton N, Berry D, Esserman L. I-SPY 2: An Adaptive Breast Cancer Trial Design in the Setting of Neoadjuvant Chemotherapy. Clin Pharmacol Ther [Internet]. 2009;86(1):97–100. Disponible en: https://doi.org/10.1038/clpt.2009.68
- ECOG-ACRIN Cancer Research Group. EAY131 / NCI-MATCH (Closed) [Internet]. Disponible en: https://ecog-acrin.org/clinical-trials/eay131-nci-match-precision-medicine/
- Azad NS, Gray RJ, Overman MJ, Schoenfeld JD, Mitchell EP, Zwiebel JA, et al. Nivolumab Is Effective in Mismatch Repair–Deficient Noncolorectal Cancers: Results From Arm Z1D—A Subprotocol of the NCI-MATCH (EAY131) Study. JCO [Internet]. 2020;38:214–22. Disponible en: https://doi.org/10.1200/jco.19.00818
- Le Tourneau C, Delord JP, Gonçalves A, Gavoille C, Dubot C, Isambert N, et al. Molecularly targeted therapy based on tumour molecular profiling versus conventional therapy for advanced cancer (SHIVA): a multicentre, open-label, proof-of-concept, randomised, controlled phase 2 trial. The Lancet Oncology [Internet]. 2015;16:1324–34. Disponible en: https://doi.org/10.1016/s1470-2045(15)00188-6
- Alva AS, Mangat PK, Garrett-Mayer E, Halabi S, Hansra D, Calfa CJ, et al. Pembrolizumab in Patients With Metastatic Breast Cancer With High Tumor Mutational Burden: Results From the Targeted Agent and Profiling Utilization Registry (TAPUR) Study. JCO [Internet]. 2021;39:2443–51. Disponible en: https://doi.org/10.1200/jco.20.02923
- Nanda R, Liu MC, Yau C, Shatsky R, Pusztai L, Wallace A, et al. Effect of Pembrolizumab Plus Neoadjuvant Chemotherapy on Pathologic Complete Response in Women With Early-Stage Breast Cancer: An Analysis of the Ongoing Phase 2 Adaptively Randomized I-SPY2 Trial. JAMA Oncol [Internet]. 2020;6:676. Disponible en: https://doi.org/10.1001/jamaoncol.2019.6650
- Gettinger SN, Redman MW, Bazhenova L, Hirsch FR, Mack PC, Schwartz LH, et al. Nivolumab Plus Ipilimumab vs Nivolumab for Previously Treated Patients With Stage IV Squamous Cell Lung Cancer: The Lung-MAP S1400I Phase 3 Randomized Clinical Trial. JAMA Oncol [Internet]. 2021;7(9):1368-1377. Disponible en: https://doi.org/10.1001/jamaoncol.2021.2209
- Vasudev NS, Scelo G, Glennon KI, Wilson M, Letourneau L, Eveleigh R, et al. Application of Genomic Sequencing to Refine Patient Stratification for Adjuvant Therapy in Renal Cell Carcinoma. Clinical Cancer Research [Internet]. 2023;29:1220–31. Disponible en: https://doi.org/10.1158/1078-0432.ccr-22-1936
- Nangalia J, Campbell PJ. Genome Sequencing during a Patient’s Journey through Cancer. N Engl J Med [Internet]. 2019;381:2145–56. Disponible en: https://doi.org/10.1056/nejmra1910138
- Winkler EC, Knoppers BM. Ethical challenges of precision cancer medicine. Seminars in Cancer Biology [Internet]. 2022;84:263–70. Disponible en: https://doi.org/10.1016/j.semcancer.2020.09.009
- Klein RD. Current Policy Challenges in Genomic Medicine. Clinical Chemistry [Internet]. 2020;66:61–7. Disponible en: https://doi.org/10.1373/clinchem.2019.308775